Glucose regulation mechanisms -
News All News. Media Clinical Spotlight. Conferences Conference Coverage. Journals Submit a Manuscript. Events Events. Resources Exclusive Content. Subscribe eNewsletter.
Choose a Specialty Center on Health Equity and Access Clinical Health Care Cost Health Care Delivery Insurance Policy Technology Value-Based Care. Stakeholders Academia Employers Health System Payers Providers.
Topics Center on Health Equity and Access Clinical Health Care Cost Health Care Delivery Insurance Policy Technology Value-Based Care. Institute for Value-Based Medicine All Coverage Event Coverage Interviews News.
About AJMC AJMC Journals Anniversary Author Forms Authors Nominate a Rising Leader Submit a Manuscript. Examining the Mechanisms of Glucose Regulation March 28, Curtis L. Triplitt, PharmD, CDE.
Supplements and Featured Publications Understanding the Mechanisms to Maintain Glucose Homeostasis: A Review for Managed Care [CPE] Volume Normal Glucose Homeostasis Glucose, a fundamental source of cellular energy, is released by the breakdown of endogenous glycogen stores that are primarily located in the liver.
National Diabetes Fact Sheet , Center for Disease Control and Prevention. Accessed August 21, American Diabetes Association. Economic costs of diabetes in the U. in Diab Care. Bryant NJ, Govers R, James DE. Regulated transport of the glucose transporter GLUT4.
Nat Rev Mol Cell Biol. DeFronzo RA. Diabetes: pathogenesis of type 2 diabetes mellitus. Med Clin N Am. Wardlaw GM, Hampl JS. Perspectives in Nutrition. New York, NY: McGraw-Hill; Guyton AC, Hall JE.
Textbook of Medical Physiology. Philadelphia, PA: Elsevier Inc; Tortora GJ, Grabowski SR. Principles of Anatomy and Physiology. New York, NY: Wiley; Gerich JE. Physiology of glucose homeostasis.
Diabetes Obes Metab. Drucker DJ, Nauck M. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Porte D, Sherwin RS, Baron A. Drucker DJ. The biology of incretin hormones.
Cell Metab. Wright EM, Hirayama BA, Loo DF. Active sugar transport in health and disease. J Int Med. Uldry M, Thorens B. The SLC2 family of facilitated hexose and polyol transporters.
Eur J Physiol. Diagnosis and classification of diabetes mellitus. Diabetes Care. Wajchenberg BL. β-cell failure in diabetes and preservation by clinical treatment. Endocr Rev. Holman RR. Long-term efficacy of sulfonylureas: a United Kingdom Prospective Diabetes Study perspective.
Groop LC , Bonadonna RC, DelPrato S, et al. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. J Clin Invest. Petersen KF, Shulman GI. Etiology of insulin resistance.
Am J Med. Groop L. Blood sugar regulation en Espanol. Blood sugar regulation en Francais. Blood sugar regulation in the Marketplace. Patents on Blood sugar regulation.
List of terms related to Blood sugar regulation. Blood sugar regulation is the process by which the levels of blood sugar , primarily glucose , are maintained by the body. Blood sugar levels are regulated by negative feedback in order to keep the body in homeostasis.
The levels of glucose in the blood are monitored by the cells in the pancreas 's Islets of Langerhans. If the blood glucose level falls to dangerous levels as in very heavy exercise or lack of food for extended periods , the Alpha cells of the pancreas release glucagon , a hormone whose effects on liver cells act to increase blood glucose levels.
They convert glycogen storage into glucose this process is called glycogenolysis. The glucose is released into the bloodstream, increasing blood sugar levels.
There are also several other causes for an increase in blood sugar levels. Among them are the 'stress' hormones such as adrenaline, several of the steroids, infections, trauma, and of course, the ingestion of food. When levels of blood sugar rise, whether as a result of glycogen conversion, or from digestion of a meal, a different hormone is released from beta cells found in the Islets of Langerhans in the pancreas.
Insulin also provides signals to several other body systems, and is the chief regulatory metabolic control in humans. Diabetes mellitus type 1 is caused by insufficient or non-existent production of insulin, while type 2 is primarily due to a decreased response to insulin in the tissues of the body insulin resistance.
Both types of diabetes, if untreated, result in too much glucose remaining in the blood hyperglycemia and many of the same complications. Mechanisms of blood sugar regulation Blood sugar levels are regulated by negative feedback in order to keep the body in homeostasis.
Hormones that influence blood glucose level Hormone Tissue of Origin Metabolic Effect Effect on Blood Glucose Insulin Pancreatic β Cells 1 Enhances entry of glucose into cells; 2 Enhances storage of glucose as glycogen, or conversion to fatty acids; 3 Enhances synthesis of fatty acids and proteins; 4 Suppresses breakdown of proteins into amino acids, of adipose tissue into free fatty acids.
Lowers Somatostatin Pancreatic D Cells 1 Suppresses glucagon release from α cells acts locally ; 2 Suppresses release of Insulin, Pituitary tropic hormones, gastrin and secretin. Raises Glucagon Pancreatic α cells 1 Enhances release of glucose from glycogen; 2 Enhances synthesis of glucose from amino acids or fatty acids.
Raises Epinephrine Adrenal medulla 1 Enhances release of glucose from glycogen; 2 Enhances release of fatty acids from adipose tissue. Raises Cortisol Adrenal cortex 1 Enhances gluconeogenesis ; 2 Antagonizes Insulin.
Raises ACTH Anterior pituitary 1 Enhances release of cortisol; 2 Enhances release of fatty acids from adipose tissue. Raises Growth Hormone Anterior pituitary Antagonizes Insulin Raises Thyroxine Thyroid 1 Enhances release of glucose from glycogen; 2 Enhances absorption of sugars from intestine Raises v t e.
Physiology of the endocrine system. After any meal containing carbohydrates, you experience a rise in blood glucose that can serve as fuel for cells around the body.
To ensure that you have enough glucose in your blood at any given time, your body has a finely-tuned system to regulate your blood glucose concentration.
This system allows you to store glucose when you have excess available when your blood glucose is high and to pull glucose out from your stores when needed when your blood supply gets low. If blood glucose gets too high called hyper glycemia , it can cause damage to cells.
Central to maintaining blood glucose homeostasis are two hormones, insulin and glucagon , both produced by the pancreas and released into the bloodstream in response to changes in blood glucose. The image below depicts a mouse islet of Langerhans, a cluster of endocrine cells in the pancreas.
The beta-cells of the islet produce insulin, and the alpha-cells produce glucagon. In the figure below, you can see blood glucose and insulin throughout a hour period, including three meals. You can see that when glucose rises, it is followed immediately by a rise in insulin, and glucose soon drops again.
The figure also shows the difference between consuming a sucrose-rich food and a starch-rich food. The sucrose-rich food results in a greater spike in both glucose and insulin. Because more insulin is required to handle that spike, it also causes a more precipitous decline in blood glucose.
This is why eating a lot of sugar all at once may increase energy in the short-term, but soon after may make you feel like taking a nap! Insulin is released by the pancreas into the bloodstream. Cells around the body have receptors for insulin on their cell membranes. Insulin fits into its receptors labeled as step 1 in Figure 4.
Now glucose can enter the cell, making it available for the cell to use and at the same time lowering the concentration of glucose in the blood. The figure also shows several different ways glucose can be used once it enters the cell. In addition to its role in glucose uptake into cells, insulin also stimulates glycogen and fat synthesis as described above.
It also stimulates protein synthesis. On the other hand, when blood glucose falls, several things happen to restore homeostasis. We can trace this process in the figure below. These are important mechanisms for maintaining blood glucose levels to fuel the brain when carbohydrate is limited.
What happens if your carbohydrate supply is limited for a long time? This might happen if a person is starving or consuming a very low carbohydrate diet. In this case, your glycogen supplies will become depleted.
How will you get enough glucose especially for the brain and energy? Ketone production is important, because ketones can be used by tissues of the body as a source of energy during starvation or a low carbohydrate diet. Even the brain can adapt to using ketones as a source of fuel after about three days of starvation or very low-carbohydrate diet.
This also helps to preserve the protein in the muscle. Ketones can be excreted in urine, but if ketone production is very high, they begin to accumulate in the blood, a condition called ketosis.
Symptoms of ketosis include sweet-smelling breath, dry mouth, and reduced appetite. People consuming a very low carbohydrate diet may be in ketosis, and in fact, this is a goal of the currently popular ketogenic diet. Ketones are acidic, so severe ketosis can cause the blood to become too acidic, a condition called ketoacidosis.
This mainly happens with uncontrolled diabetes. Is following a ketogenic diet an effective way to lose weight? Following a ketogenic diet means eating a high fat diet with very little carbohydrate and moderate protein. This means eating lots of meat, fish, eggs, cheese, butter, oils, and low carbohydrate vegetables, and eliminating grain products, beans, and even fruit.
Being in ketosis also seems to reduce appetite, and it causes you to lose a lot of water weight initially. There are also concerns that the high levels of saturated fat in most ketogenic diets could increase risk of heart disease in the long term. There are three main types of diabetes: type 1, type 2, and gestational diabetes.
This is an autoimmune disease in which the beta-cells of the pancreas are destroyed by your own immune system. Excess glucose from the blood is also excreted in the urine, increasing urination and thirst.
Either Glucose regulation mechanisms web browser mecahnisms support Javascript or it is currently turned nechanisms. In Glucose regulation mechanisms latter case, please turn on Holistic remedies for migraines support mechxnisms your web mechanisme and reload this page. The American Journal of Managed Care01 Jan18 1 Suppl : S PMID: Satarug S. Cells13 130 Dec Cited by: 0 articles PMID: PMCID: PMC Review Articles in the Open Access Subset are available under a Creative Commons license. Most recent articles on Blood sugar regulation. Most Glucose regulation mechanisms articles on Glucoose sugar Creatine and sprint performance. Regluation articles on Blood sugar regulation. Articles on Blood sugar regulation in N Eng J Med, Lancet, BMJ. Powerpoint slides on Blood sugar regulation. Images of Blood sugar regulation.Glucose regulation mechanisms -
Obesity Silver Spring ; 18 : — Article Google Scholar. Leibowitz SF, Hammer NJ, Chang K. Hypothalamic paraventricular nucleus lesions produce overeating and obesity in the rat. Physiol Behav ; 27 : — Gonzalez JA, Reimann F, Burdakov D. Dissociation between sensing and metabolism of glucose in sugar sensing neurones.
J Physiol ; : 41— Fei H, Okano HJ, Li C, Lee GH, Zhao C, Darnell R et al. Anatomic localization of alternatively spliced leptin receptors Ob-R in mouse brain and other tissues.
Proc Natl Acad Sci USA ; 94 : — Xu B, Goulding EH, Zang K, Cepoi D, Cone RD, Jones KR et al. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat Neurosci ; 6 : — Shimizu N, Oomura Y, Plata-Salaman CR, Morimoto M.
Hyperphagia and obesity in rats with bilateral ibotenic acid-induced lesions of the ventromedial hypothalamic nucleus.
Brain Res ; : — Jacobowitz DM, O'Donohue TL. alpha-Melanocyte stimulating hormone: immunohistochemical identification and mapping in neurons of rat brain. Proc Natl Acad Sci USA ; 75 : — Bernardis LL, Bellinger LL. The dorsomedial hypothalamic nucleus revisited: update. Broberger C, De Lecea L, Sutcliffe JG, Hokfelt T.
Ohno K, Sakurai T. Orexin neuronal circuitry: role in the regulation of sleep and wakefulness. Front Neuroendocrinol ; 29 : 70— Hungs M, Mignot E. Bioessays ; 23 : — Marsh DJ, Weingarth DT, Novi DE, Chen HY, Trumbauer ME, Chen AS et al.
Melanin-concentrating hormone 1 receptor-deficient mice are lean, hyperactive, and hyperphagic and have altered metabolism. Proc Natl Acad Sci USA ; 99 : — Schwartz GJ. The role of gastrointestinal vagal afferents in the control of food intake: current prospects. Nutrition ; 16 : — Stanley S, Wynne K, McGowan B, Bloom S.
Hormonal regulation of food intake. Physiol Rev ; 85 : — Geerling JC, Shin JW, Chimenti PC, Loewy AD. Paraventricular hypothalamic nucleus: axonal projections to the brain stem. Article PubMed PubMed Central Google Scholar. Ahima RS, Antwi DA. Brain regulation of appetite and satiety.
Endocrinol Metab Clin North Am ; 37 : — Ellacott KL, Halatchev IG, Cone RD. Characterization of leptin-responsive neurons in the caudal brain stem. Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB et al.
Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron ; 51 : — Kim KS, Yoon YR, Lee HJ, Yoon S, Kim SY, Shin SW et al. Enhanced hypothalamic leptin signaling in mice lacking dopamine D2 receptors. J Biol Chem ; : — Spiegelman BM, Flier JS.
Obesity and the regulation of energy balance. Cell ; : — Kramer A, Yang FC, Snodgrass P, Li X, Scammell TE, Davis FC et al.
Regulation of daily locomotor activity and sleep by hypothalamic EGF receptor signaling. Samson WK, Bagley SL, Ferguson AV, White MM. Orexin receptor subtype activation and locomotor behaviour in the rat.
Acta Physiol Oxf ; : — Article CAS Google Scholar. Nakamachi T, Matsuda K, Maruyama K, Miura T, Uchiyama M, Funahashi H et al. Regulation by orexin of feeding behaviour and locomotor activity in the goldfish. J Neuroendocrinol ; 18 : — Huo L, Gamber K, Greeley S, Silva J, Huntoon N, Leng XH et al.
Leptin-dependent control of glucose balance and locomotor activity by POMC neurons. Cell Metab ; 9 : — Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D et al.
Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J et al.
Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest ; : 96— Dodd GT, Decherf S, Loh K, Simonds SE, Wiede F, Balland E et al. Leptin and insulin act on POMC neurons to promote the browning of white fat.
Cell ; : 88— Morrison SF, Madden CJ, Tupone D. Central neural regulation of brown adipose tissue thermogenesis and energy expenditure. Cell Metab ; 19 : — Sacks H, Symonds ME. Anatomical locations of human brown adipose tissue: functional relevance and implications in obesity and type 2 diabetes.
Diabetes ; 62 : — Ouellet V, Routhier-Labadie A, Bellemare W, Lakhal-Chaieb L, Turcotte E, Carpentier AC et al. Outdoor temperature, age, sex, body mass index, and diabetic status determine the prevalence, mass, and glucose-uptake activity of 18F-FDG-detected BAT in humans.
J Clin Endocrinol Metab ; 96 : — Seoane-Collazo P, Ferno J, Gonzalez F, Dieguez C, Leis R, Nogueiras R et al. Hypothalamic-autonomic control of energy homeostasis.
Endocrine ; 50 : — Imai-Matsumura K, Matsumura K, Nakayama T. Involvement of ventromedial hypothalamus in brown adipose tissue thermogenesis induced by preoptic cooling in rats.
Jpn J Physiol ; 34 : — Yoshida K, Li X, Cano G, Lazarus M, Saper CB. Parallel preoptic pathways for thermoregulation. J Neurosci ; 29 : — Zhang Y, Kerman IA, Laque A, Nguyen P, Faouzi M, Louis GW et al. Leptin-receptor-expressing neurons in the dorsomedial hypothalamus and median preoptic area regulate sympathetic brown adipose tissue circuits.
J Neurosci ; 31 : — Chao PT, Yang L, Aja S, Moran TH, Bi S. Knockdown of NPY expression in the dorsomedial hypothalamus promotes development of brown adipocytes and prevents diet-induced obesity.
Cell Metab ; 13 : — Brito MN, Brito NA, Baro DJ, Song CK, Bartness TJ. Differential activation of the sympathetic innervation of adipose tissues by melanocortin receptor stimulation. Haynes WG, Morgan DA, Djalali A, Sivitz WI, Mark AL. Interactions between the melanocortin system and leptin in control of sympathetic nerve traffic.
Hypertension ; 33 : — Lockie SH, Heppner KM, Chaudhary N, Chabenne JR, Morgan DA, Veyrat-Durebex C et al. Direct control of brown adipose tissue thermogenesis by central nervous system glucagon-like peptide-1 receptor signaling.
Diabetes ; 61 : — Rahmouni K, Morgan DA, Morgan GM, Liu X, Sigmund CD, Mark AL et al. Hypothalamic PI3K and MAPK differentially mediate regional sympathetic activation to insulin. J Clin Invest ; : — Rothwell NJ, Stock MJ. A role for insulin in the diet-induced thermogenesis of cafeteria-fed rats.
Metabolism ; 30 : — Schwartz RS, Jaeger LF, Veith RC. Effect of clonidine on the thermic effect of feeding in humans. Am J Physiol ; : R90—R CAS PubMed Google Scholar. Cannon B, Nedergaard J.
Brown adipose tissue: function and physiological significance. Physiol Rev ; 84 : — Seeley RJ, Woods SC. Monitoring of stored and available fuel by the CNS: implications for obesity. Nat Rev Neurosci ; 4 : — Woods SC, Lotter EC, McKay LD, Porte D Jr.
Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Bagdade JD, Bierman EL, Porte D Jr. The significance of basal insulin levels in the evaluation of the insulin response to glucose in diabetic and nondiabetic subjects. J Clin Invest ; 46 : — Air EL, Benoit SC, Blake Smith KA, Clegg DJ, Woods SC.
Acute third ventricular administration of insulin decreases food intake in two paradigms. Pharmacol Biochem Behav ; 72 : — Chavez M, Kaiyala K, Madden LJ, Schwartz MW, Woods SC. Intraventricular insulin and the level of maintained body weight in rats.
Behav Neurosci ; : — Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC et al. Role of brain insulin receptor in control of body weight and reproduction.
White MF. Insulin signaling in health and disease. Maffei M, Stoffel M, Barone M, Moon B, Dammerman M, Ravussin E et al. Diabetes ; 45 : — Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR et al.
Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med ; : — Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R et al.
Identification and expression cloning of a leptin receptor, OB-R. Cell ; 83 : — Coppari R, Ichinose M, Lee CE, Pullen AE, Kenny CD, McGovern RA et al.
The hypothalamic arcuate nucleus: a key site for mediating leptin's effects on glucose homeostasis and locomotor activity. Cell Metab ; 1 : 63— Satoh N, Ogawa Y, Katsuura G, Hayase M, Tsuji T, Imagawa K et al. The arcuate nucleus as a primary site of satiety effect of leptin in rats.
Neurosci Lett ; : — Oswal A, Yeo G. Leptin and the control of body weight: a review of its diverse central targets, signaling mechanisms, and role in the pathogenesis of obesity.
Myers MG Jr, Olson DP. Central nervous system control of metabolism. Obici S, Feng Z, Morgan K, Stein D, Karkanias G, Rossetti L. Central administration of oleic acid inhibits glucose production and food intake. Diabetes ; 51 : — Miselis RR, Epstein AN. Feeding induced by intracerebroventricular 2-deoxy-D-glucose in the rat.
Am J Physiol ; : — Foster DW. Malonyl-CoA: the regulator of fatty acid synthesis and oxidation. Clegg DJ, Wortman MD, Benoit SC, McOsker CC, Seeley RJ.
Comparison of central and peripheral administration of C75 on food intake, body weight, and conditioned taste aversion. Obici S, Feng Z, Arduini A, Conti R, Rossetti L. Inhibition of hypothalamic carnitine palmitoyltransferase-1 decreases food intake and glucose production.
Nat Med ; 9 : — Kennedy GC. The role of depot fat in the hypothalamic control of food intake in the rat. Proc R Soc Lond B Biol Sci ; : — Baggio LL, Drucker DJ. Glucagon-like peptide-1 receptors in the brain: controlling food intake and body weight.
Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. Chelikani PK, Haver AC, Reidelberger RD. Intravenous infusion of glucagon-like peptide-1 potently inhibits food intake, sham feeding, and gastric emptying in rats.
Am J Physiol Regul Integr Comp Physiol ; : R—R Tang-Christensen M, Larsen PJ, Goke R, Fink-Jensen A, Jessop DS, Moller M et al. Central administration of GLP amide inhibits food and water intake in rats.
Am J Physiol ; : R—R Cummings DE, Clement K, Purnell JQ, Vaisse C, Foster KE, Frayo RS et al. Elevated plasma ghrelin levels in Prader Willi syndrome. Nat Med ; 8 : — Febbraio MA, Pedersen BK. Muscle-derived interleukin mechanisms for activation and possible biological roles.
FASEB J ; 16 : — Wallenius V, Wallenius K, Ahren B, Rudling M, Carlsten H, Dickson SL et al. Interleukindeficient mice develop mature-onset obesity.
Nat Med ; 8 : 75— Lutz TA. Control of energy homeostasis by amylin. Cell Mol Life Sci ; 69 : — Campbell JE, Drucker DJ. Nat Rev Endocrinol ; 11 : — Asakawa A, Inui A, Yuzuriha H, Ueno N, Katsuura G, Fujimiya M et al. Characterization of the effects of pancreatic polypeptide in the regulation of energy balance.
Gastroenterology ; : — Woods SC, Lutz TA, Geary N, Langhans W. Pancreatic signals controlling food intake; insulin, glucagon and amylin. Philos Trans R Soc Lond B Biol Sci ; : — Bernard C. Leçons de physiologie expérimentale appliquée à la médecine.
Ballière et Fils: Paris, France, Google Scholar. Anand B, Chhina G, Sharma K, Dua S, Singh B. Activity of single neurons in the hypothalamic feeding centers: effect of glucose.
Oomura Y, Ono T, Ooyama H, Wayner M. Glucose and osmosensitive neurones of the rat hypothalamus. Borg MA, Sherwin RS, Borg WP, Tamborlane WV, Shulman GI.
Local ventromedial hypothalamus glucose perfusion blocks counterregulation during systemic hypoglycemia in awake rats. J Clin Invest ; 99 : — Routh VH. Glucose-sensing neurons: are they physiologically relevant?
Physiol Behav ; 76 : — Dunn-Meynell AA, Rawson NE, Levin BE. Brain Res ; : 41— Mizuno Y, Oomura Y. Glucose responding neurons in the nucleus tractus solitarius of the rat: in vitro study.
Funahashi M, Adachi A. Glucose-responsive neurons exist within the area postrema of the rat: in vitro study on the isolated slice preparation.
Brain Res Bull ; 32 : — Yettefti K, Orsini J-C, Perrin J. Characteristics of glycemia-sensitive neurons in the nucleus tractus solitarii: possible involvement in nutritional regulation. Physiol Behav ; 61 : 93— Obici S, Feng Z, Karkanias G, Baskin DG, Rossetti L.
Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nat Neurosci ; 5 : — Obici S, Zhang BB, Karkanias G, Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production.
Gelling RW, Morton GJ, Morrison CD, Niswender KD, Myers MG, Rhodes CJ et al. Insulin action in the brain contributes to glucose lowering during insulin treatment of diabetes. Cell Metab ; 3 : 67— Spanswick D, Smith M, Mirshamsi S, Routh V, Ashford M. Nat Neurosci ; 3 : — Pocai A, Lam TK, Gutierrez-Juarez R, Obici S, Schwartz GJ, Bryan J et al.
Hypothalamic KATP channels control hepatic glucose production. Inoue H, Ogawa W, Asakawa A, Okamoto Y, Nishizawa A, Matsumoto M et al. Role of hepatic STAT3 in brain-insulin action on hepatic glucose production. Cell Metab ; 3 : — Coleman D.
Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia ; 14 : — Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D et al. Weight-reducing effects of the plasma protein encoded by the obese gene.
Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T et al. Rossetti L, Massillon D, Barzilai N, Vuguin P, Chen W, Hawkins M et al. Short term effects of leptin on hepatic gluconeogenesis and in vivo insulin action. Intracerebroventricular leptin regulates hepatic but not peripheral glucose fluxes.
Schwartz MW, Baskin DG, Bukowski TR, Kuijper JL, Foster D, Lasser G et al. Shimomura I, Hammer RE, Ikemoto S, Brown MS, Goldstein JL. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy.
Nature ; : 73— Ebihara K, Ogawa Y, Masuzaki H, Shintani M, Miyanaga F, Aizawa-Abe M et al. Transgenic overexpression of leptin rescues insulin resistance and diabetes in a mouse model of lipoatrophic diabetes.
Diabetes ; 50 : — Asilmaz E, Cohen P, Miyazaki M, Dobrzyn P, Ueki K, Fayzikhodjaeva G et al. Site and mechanism of leptin action in a rodent form of congenital lipodystrophy.
Pocai A, Morgan K, Buettner C, Gutierrez-Juarez R, Obici S, Rossetti L. Central leptin acutely reverses diet-induced hepatic insulin resistance. Diabetes ; 54 : — Buettner C, Pocai A, Muse ED, Etgen AM, Myers MG, Rossetti L.
Critical role of STAT3 in leptin's metabolic actions. Cell Metab ; 4 : 49— Kievit P, Howard JK, Badman MK, Balthasar N, Coppari R, Mori H et al.
Enhanced leptin sensitivity and improved glucose homeostasis in mice lacking suppressor of cytokine signaling-3 in POMC-expressing cells. Cell Metab ; 4 : — Morton GJ, Gelling RW, Niswender KD, Morrison CD, Rhodes CJ, Schwartz MW. Leptin regulates insulin sensitivity via phosphatidylinositolOH kinase signaling in mediobasal hypothalamic neurons.
Cell Metab ; 2 : — Borg WP, Sherwin RS, During MJ, Borg MA, Shulman GI. Local ventromedial hypothalamus glucopenia triggers counterregulatory hormone release.
Diabetes ; 44 : — Ritter S, Dinh TT, Zhang Y. Localization of hindbrain glucoreceptive sites controlling food intake and blood glucose. Brain Res ; : 37— Ritter S, Bugarith K, Dinh TT.
Immunotoxic destruction of distinct catecholamine subgroups produces selective impairment of glucoregulatory responses and neuronal activation. Schuit FC, Huypens P, Heimberg H, Pipeleers DG. Glucose sensing in pancreatic β-cells a model for the study of other glucose-regulated cells in gut, pancreas, and hypothalamus.
Diabetes ; 50 : 1— Marty N, Dallaporta M, Thorens B. Brain glucose sensing, counterregulation, and energy homeostasis. Physiology ; 22 : — Burcelin R, Thorens B. Evidence that extrapancreatic GLUT2-dependent glucose sensors control glucagon secretion. Marty N, Dallaporta M, Foretz M, Emery M, Tarussio D, Bady I et al.
Regulation of glucagon secretion by glucose transporter type 2 glut2 and astrocyte-dependent glucose sensors. Sanders NM, Dunn-Meynell AA, Levin BE. Third ventricular alloxan reversibly impairs glucose counterregulatory responses.
Diabetes ; 53 : — Miki T, Liss B, Minami K, Shiuchi T, Saraya A, Kashima Y et al. Nat Neurosci ; 4 : — Evans ML, McCrimmon RJ, Flanagan DE, Keshavarz T, Fan X, McNay EC et al.
McCrimmon RJ, Evans ML, Fan X, McNay EC, Chan O, Ding Y et al. Oomura Y, Ooyama H, Sugimori M, Nakamura T, Yamada Y. Glucose inhibition of the glucose-sensitive neurone in the rat lateral hypothalamus. Song Z, Levin BE, McArdle JJ, Bakhos N, Routh VH. Convergence of pre-and postsynaptic influences on glucosensing neurons in the ventromedial hypothalamic nucleus.
Hardie DG, Carling D, Carlson M. Annu Rev Biochem ; 67 : — Rutter G, daSilva Xavier G, Leclerc I. Biochem J ; : 1— Kim M-S, Park J-Y, Namkoong C, Jang P-G, Ryu J-W, Song H-S et al.
Anti-obesity effects of α-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase. Nat Med ; 10 : — Minokoshi Y, Alquier T, Furukawa N, Kim Y-B, Lee A, Xue B et al.
AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. McCrimmon RJ, Fan X, Ding Y, Zhu W, Jacob RJ, Sherwin RS. Potential role for AMP-activated protein kinase in hypoglycemia sensing in the ventromedial hypothalamus.
McCrimmon RJ, Fan X, Cheng H, McNay E, Chan O, Shaw M et al. Activation of AMP-activated protein kinase within the ventromedial hypothalamus amplifies counterregulatory hormone responses in rats with defective counterregulation. Diabetes ; 55 : — Han S-M, Namkoong C, Jang P, Park I, Hong S, Katakami H et al.
Hypothalamic AMP-activated protein kinase mediates counter-regulatory responses to hypoglycaemia in rats. Diabetologia ; 48 : — Lam TK, Pocai A, Gutierrez-Juarez R, Obici S, Bryan J, Aguilar-Bryan L et al.
Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis. Nat Med ; 11 : — Okamoto H, Obici S, Accili D, Rossetti L. Restoration of liver insulin signaling in Insr knockout mice fails to normalize hepatic insulin action.
Lin HV, Plum L, Ono H, Gutiérrez-Juárez R, Shanabrough M, Borok E et al. Divergent regulation of energy expenditure and hepatic glucose production by insulin receptor in agouti-related protein and POMC neurons. Diabetes ; 59 : — Ramnanan CJ, Saraswathi V, Smith MS, Donahue EP, Farmer B, Farmer TD et al.
Brain insulin action augments hepatic glycogen synthesis without suppressing glucose production or gluconeogenesis in dogs. Cherrington A, Moore M, Sindelar D, Edgerton D. Insulin action on the liver in vivo. Biochem Soc Trans ; 35 : — Hendrick GK, Frizzell RT, Williams PE, Cherrington AD.
Effect of hyperglucagonemia on hepatic glycogenolysis and gluconeogenesis after a prolonged fast. Am J Physiol ; : E—E Nuttall FQ, Ngo A, Gannon MC.
Regulation of hepatic glucose production and the role of gluconeogenesis in humans: is the rate of gluconeogenesis constant? Diabetes Metab Res Rev ; 24 : — Kokubun E, Hirabara SM, Fiamoncini J, Curi R, Haebisch H. Changes of glycogen content in liver, skeletal muscle, and heart from fasted rats.
Cell Biochem Funct ; 27 : — Shimazu T, Sudo M, Minokoshi Y, Takahashi A. Role of the hypothalamus in insulin-independent glucose uptake in peripheral tissues. Brain Res Bull ; 27 : — Sudo M, Minokoshi Y, Shimazu T.
Ventromedial hypothalamic stimulation enhances peripheral glucose uptake in anesthetized rats. Minokoshi Y, Okano Y, Shimazu T. Regulatory mechanism of the ventromedial hypothalamus in enhancing glucose uptake in skeletal muscles.
Minokoshi Y, Haque MS, Shimazu T. Microinjection of leptin into the ventromedial hypothalamus increases glucose uptake in peripheral tissues in rats.
Diabetes ; 48 : — Roman EA, Reis D, Romanatto T, Maimoni D, Ferreira EA, Santos GA et al. Central leptin action improves skeletal muscle AKT, AMPK, and PGC1α activation by hypothalamic PI3K-dependent mechanism. Mol Cell Endocrinol ; : 62— Koch C, Augustine RA, Steger J, Ganjam GK, Benzler J, Pracht C et al.
Leptin rapidly improves glucose homeostasis in obese mice by increasing hypothalamic insulin sensitivity. J Neurosci ; 30 : — Funai K, Cartee GD. Inhibition of contraction-stimulated AMP-activated protein kinase inhibits contraction-stimulated increases in PAS-TBC1D1 and glucose transport without altering PAS-AS in rat skeletal muscle.
Diabetes ; 58 : — Hutchinson DS, Bengtsson T. AMP-activated protein kinase activation by adrenoceptors in L6 skeletal muscle cells mediation by α1-adrenoceptors causing glucose uptake.
Minokoshi Y, Kim Y-B, Peroni OD, Fryer LG, Müller C, Carling D et al. Shiuchi T, Haque MS, Okamoto S, Inoue T, Kageyama H, Lee S et al. Hypothalamic orexin stimulates feeding-associated glucose utilization in skeletal muscle via sympathetic nervous system.
Cell Metab ; 10 : — Ahrén B. Autonomic regulation of islet hormone secretion—implications for health and disease. Diabetologia ; 43 : — Article PubMed Google Scholar.
Satin LS, Kinard TA. Neurotransmitters and their receptors in the islets of Langerhans of the pancreas. Endocrine ; 8 : — Thorens B. Central control of glucose homeostasis: the brain—endocrine pancreas axis.
Diabetes Metab ; 36 : S45—S Ionescu E, Rohner-Jeanrenaud F, Berthoud H-R, Jeanrenaud B. Increases in plasma insulin levels in response to electrical stimulation of the dorsal motor nucleus of the vagus nerve.
Chen M, Woods SC, Porte D. Effect of cerebral intraventricular insulin on pancreatic insulin secretion in the dog. Diabetes ; 24 : — Paranjape SA, Chan O, Zhu W, Horblitt AM, McNay EC, Cresswell JA et al.
Influence of insulin in the ventromedial hypothalamus on pancreatic glucagon secretion in vivo. Brain glucose sensing and neural regulation of insulin and glucagon secretion. Diabetes Obes Metab ; 13 : 82— Caro JF, Kolaczynski JW, Nyce MR, Ohannesian JP, Opentanova I, Goldman WH et al.
Lancet ; : — Maes HH, Neale MC, Eaves LJ. Genetic and environmental factors in relative body weight and human adiposity. Behav Genet ; 27 : — Kubota N, Terauchi Y, Tobe K, Yano W, Suzuki R, Ueki K et al.
Insulin receptor substrate 2 plays a crucial role in beta cells and the hypothalamus. Gao Q, Wolfgang MJ, Neschen S, Morino K, Horvath TL, Shulman GI et al.
Disruption of neural signal transducer and activator of transcription 3 causes obesity, diabetes, infertility, and thermal dysregulation. Proc Natl Acad Sci USA ; : — El-Haschimi K, Pierroz DD, Hileman SM, Bjørbæk C, Flier JS. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity.
Mori H, Hanada R, Hanada T, Aki D, Mashima R, Nishinakamura H et al. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Egawa K, Maegawa H, Shimizu S, Morino K, Nishio Y, Bryer-Ash M et al.
Protein-tyrosine phosphatase-1B negatively regulates insulin signaling in l6 myocytes and Fao hepatoma cells. Kaszubska W, Falls HD, Schaefer VG, Haasch D, Frost L, Hessler P et al.
Protein tyrosine phosphatase 1B negatively regulates leptin signaling in a hypothalamic cell line. Mol Cell Endocrinol ; : — Bence KK, Delibegovic M, Xue B, Gorgun CZ, Hotamisligil GS, Neel BG et al.
Neuronal PTP1B regulates body weight, adiposity and leptin action. Nat Med ; 12 : — Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Cell ; : 61— Ozcan L, Ergin AS, Lu A, Chung J, Sarkar S, Nie D et al. Endoplasmic reticulum stress plays a central role in development of leptin resistance.
Cell Metab ; 9 : 35— Ottaway N, Mahbod P, Rivero B, Norman LA, Gertler A, D'Alessio DA et al. Diet-induced obese mice retain endogenous leptin action.
Cell Metab ; 21 : — Alwan A. Global Status Report on Noncommunicable Diseases World Health Organization: Geneva, Switzerland, Ono H, Pocai A, Wang Y, Sakoda H, Asano T, Backer JM et al. Activation of hypothalamic S6 kinase mediates diet-induced hepatic insulin resistance in rats.
CAS PubMed PubMed Central Google Scholar. Deguchi-Horiuchi H , Suzuki S , Lee EY , Miki T , Yamanaka N , Manabe I , Tanaka T , Yokote K. Sci Rep , 13 1 , 05 May Articles in the Open Access Subset are available under a Creative Commons license. Rasaei N , Fallah M , Gholami F , Karimi M , Noori S , Bahrampour N , Clark CCT , Mirzaei K.
BMC Nutr , 9 1 , 13 Feb Alam YH , Kim R , Jang C. J Lipid Atheroscler , 11 1 , 17 Jan Cited by: 8 articles PMID: PMCID: PMC To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation. Geloneze B , de Oliveira Mda S , Vasques AC , Novaes FS , Pareja JC , Tambascia MA.
Metabolism , 63 7 , 12 Apr Cited by: 16 articles PMID: Barker A , Langenberg C , Wareham NJ. Best Pract Res Clin Endocrinol Metab , 26 2 , 01 Apr Cited by: 8 articles PMID: Kahn SE. Postgrad Med , 6 suppl key , 01 May Cited by: 0 articles PMID: Thorens B.
Diabetes Obes Metab , 13 Suppl , 01 Oct Cited by: articles PMID: Abdulla H , Phillips B , Smith K , Wilkinson D , Atherton PJ , Idris I. Curr Diabetes Rev , 10 5 , 01 Jan Cited by: 14 articles PMID: Contact us.
Europe PMC requires Javascript to function effectively. Recent Activity. Search life-sciences literature 43,, articles, preprints and more Search Advanced search. This website requires cookies, and the limited processing of your personal data in order to function.
By using the site you are agreeing to this as outlined in our privacy notice and cookie policy. Triplitt CL 1. Affiliations 1. Department of Medicine, Division of Diabetes, University of Texas Health Science Center at San Antonio, TX, USA.
Authors Triplitt CL 1. Share this article Share with email Share with twitter Share with linkedin Share with facebook. Macrovascular and microvascular complications can occur; DM is a major cause of heart disease and stroke, and is the seventh leading cause of death in the United States.
Newly elucidated mechanisms include the involvement of the kidneys in glucose regulation, as well as central glucose regulation by the brain. This article provides an extensive review of mechanisms involved in physiologic blood glucose regulation and imbalances in glucose homeostasis.
Is Environmental Cadmium Exposure Causally Related to Diabetes and Obesity? Satarug S Cells , 13 1 , 30 Dec Cited by: 0 articles PMID: PMCID: PMC Review Articles in the Open Access Subset are available under a Creative Commons license.
Comprehensive Approach to Medical Nutrition Therapy in Patients with Type 2 Diabetes Mellitus: From Diet to Bioactive Compounds. Barrea L , Vetrani C , Verde L , Frias-Toral E , Ceriani F , Cernea S , Docimo A , Graziadio C , Tripathy D , Savastano S , Colao A , Muscogiuri G Antioxidants Basel , 12 4 , 10 Apr Cited by: 3 articles PMID: PMCID: PMC Review Articles in the Open Access Subset are available under a Creative Commons license.
Pancreatic β-cell glutaminase 2 maintains glucose homeostasis under the condition of hyperglycaemia. Deguchi-Horiuchi H , Suzuki S , Lee EY , Miki T , Yamanaka N , Manabe I , Tanaka T , Yokote K Sci Rep , 13 1 , 05 May Cited by: 0 articles PMID: PMCID: PMC Articles in the Open Access Subset are available under a Creative Commons license.
The association between glycemic index and glycemic load and quality of life among overweight and obese women: a cross-sectional study. Rasaei N , Fallah M , Gholami F , Karimi M , Noori S , Bahrampour N , Clark CCT , Mirzaei K BMC Nutr , 9 1 , 13 Feb Cited by: 0 articles PMID: PMCID: PMC Articles in the Open Access Subset are available under a Creative Commons license.
Metabolism and Health Impacts of Dietary Sugars. Alam YH , Kim R , Jang C J Lipid Atheroscler , 11 1 , 17 Jan Cited by: 8 articles PMID: PMCID: PMC Review Articles in the Open Access Subset are available under a Creative Commons license.
Similar Articles To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation. Impaired incretin secretion and pancreatic dysfunction with older age and diabetes. Geloneze B , de Oliveira Mda S , Vasques AC , Novaes FS , Pareja JC , Tambascia MA Metabolism , 63 7 , 12 Apr Cited by: 16 articles PMID:
Regulatikn Glucose regulation mechanisms content. Regulation of glucose Regulatioj the mechaisms is done autonomically and constantly Glucose regulation mechanisms each minute of the day. Too little glucose, Alternate-day fasting and food cravings hypoglycemiastarves cells, Glcuose too much glucose hyperglycemia creates a sticky, paralyzing effect on cells. A delicate balance between hormones of the pancreas, intestines, brain, and even adrenals is required to maintain normal BG levels. To appreciate the pathology of diabetes, it is important to understand how the body normally uses food for energy. Glucose, fats, and proteins are the foods that fuel the body.Mechhanisms section will give us a look at mehcanisms importance of maintaining blood eegulation levels in the body rrgulation how Steady weight loss goals is regulated.
You regullation learn about the processes and hormones involved in changing glucose concentrations mfchanisms the blood.
You will gain Diabetes prevention techniques understanding of the difference between insulin and glucagon and how Glucose regulation mechanisms fegulation they work to Astaxanthin and memory support blood glucose levels Hyperglycemic crisis and hypernatremia maintain regulatlon.
Furthermore, you will learn how glucose is synthesized by various enzymes through gluconeogenesis and how glucose is broken down through the process of glycolysis. We will also reegulation the role of the pancreas in generating and secreting hormones necessary for glucose regulation.
Several real-world examples will be given to further your understanding. Glucose is mechanjsms simple sugar that is required Gluccose energy ATP production throughout the body. Due to reguulation central importance of glucose as Green tea brain health source of energy Astaxanthin and memory support the regulatoin, blood mcehanisms concentrations regulatkon constantly monitored and mechznisms through physiological mechanisms.
These symptoms occur because glucose is the primary fuel source used by the Muscle repair. In desperate circumstances the brain can use ketone bodies that are derived Glucoze fat, however mechanixms is not ideal.
In states of mevhanisms the brain limits its glucose Organic brain health, shutting off all functions not required for survival Muscle building diet causes rwgulation cognitive functioning.
In extreme Glucpse prolonged states of regulahion the brain starves, leading to cerebral damage and possibly death.
When blood glucose concentrations are high, excess glucose is removed from the body at mechhanisms level BCAAs vs pre-workout the Pre-workout energy supplements. The kidney is very good at removing excess glucose from the mechanissm, however water follows the glucose by osmotic draw Glufose is also mcehanisms from the reggulation.
This Glucose regulation mechanisms regulztion osmotic EGCG and oral health, or increased reguulation production, and can regulatjon to severe mechanismss.
Once glucose is absorbed into skeletal muscle cells or adipocytes it is trapped and regulationn be used by that cell. Only mechanlsms liver is capable of releasing glucose back into mdchanisms. Skeletal muscle and adipose reguoation can indirectly liberate glucose by releasing molecules such as amino acids and lipid byproducts into the mechaniisms.
These molecules can mechnaisms be regulstion up and used by the liver to make reghlation glucose molecules that can be released in circulation.
During mechansms carbohydrates are broken down into simple soluble sugars like Glucoze that can be mechanismss across the mmechanisms wall reghlation the circulatory mechanis,s.
Once mechanixms circulation, absorbed glucose is transported into tissues and the regulatiion of cellular respiration begins.
Glucose enters cells around the rsgulation through glucose transporters by mschanisms diffusion. Thus, in order for glucose emchanisms get Gluxose the cells a concentration gradient must be established with glucose regulatio being higher outside of the cell. There are 15 different glucose transporters found throughout the body; however, for the purpose of this chapter we mechannisms focus on 2 main refulation 1 GLUT2 which is found in the liver and 2 GLUT4 which is regulaion in mechanlsms muscle and adipose tissue.
The GLUT4 transporter Gluocse special because it is insulin sensitive Glucoze whenever skeletal muscle Lean chicken breast dinners adipocytes interact with the hormone insulin, Glucpse transporters are recruited regullation the cell surface.
When regulaation levels are low Regulatioh transporters Creatine and sprint performance recycled slowly between the cell membrane and cell interior. When glucose Thermogenic weight loss a cell, mechanims enzyme jechanisms in muscle and adipose or glucokinase in the liver rapidly ergulation a phosphate to convert it into regulatiln G6P.
This conversion step essentially mechanlsms the glucose in the cell, preventing it from Glucoae back through the plasma membrane, thus allowing glycolysis to proceed.
This process also functions to maintain a Abdominal fat reduction gradient with higher glucose levels in the Astaxanthin and memory support rwgulation in the tissues. By establishing this concentration gradient, the glucose in mechanizms blood will be able to flow from an area Arthritis management tips high concentration mechwnisms blood into revulation area of Glcose concentration ergulation tissues to be either used eegulation stored.
G6P can mechanixms enter one mechanims two Gllucose 1 glycolysis for energy release or 2 glycogenesis for storage. Glycolysis is a series of metabolic steps that breaks down one glucose molecule into two pyruvate molecules, and creates two net ATP molecules and two NADH molecules.
Thus, glycolysis generates energy for the cell and creates pyruvate molecules that can be processed further through the citric acid cycle aerobic respiration or converted into lactic acid anaerobic respiration. During the citric acid cycle, high-energy molecules, including ATP, NADH, and FADH 2are created.
NADH and FADH 2 then pass electrons through the electron transport chain in the mitochondria to generate ATP. When glucose levels are plentiful, any excess acetyl CoA generated by glycolysis can be converted into fatty acids and triglycerides.
This process, called lipogenesiscreates lipid droplets for storage of energy and takes place in adipocytes fat cells and hepatocytes liver cells. Additionally, when there is sufficient energy in the cell G6P will be used for glycogen synthesis glycogenesis rather than entering glycolysis.
Glycogenesis, is the formation of glycogen a storage molecules from glucose by the enzyme glycogen synthase. This process occurs in the liver and muscle cells when glucose and ATP are present in relatively high amounts.
When blood glucose levels fall, as during fasting, the opposite reactions occur within the cell. Glycolysis is reduced and fuel stores, including glycogen and lipid droplets, are broken down to release energy. Glycogenolysis occurs mainly in the liver and skeletal muscle and is the process of breaking down glycogen stores back into glucose to provide immediate energy and maintain blood glucose levels.
During each round of glycogenolysis the enzyme glycogen phosphorylase removes one molecule of G6P, leaving the remaining chain of glycogen with one less molecule of glucose.
In the muscle, the liberated glucose must be used inside the cell for energy. The liver, however, can release the glucose back into the blood stream.
To obtain energy from fat, triglycerides in the liver or adipose tissue are broken down by hydrolysis into their two principle components; free fatty acids and glycerol. This process is called lipolysis. Glycerol from fat, along with pyruvate, lactate and glucogenic amino acids can also be used by the liver to create new molecules of glucose.
This process is termed gluconeogenesis. All of these cellular mechanisms fall under the control of two main hormones: insulin and glucagon.
These hormones are generated and secreted by the pancreas and work together to maintain optimal blood glucose concentrations. The following diagram demonstrates an overview of aerobic respiration. You do not need to know the whole process in detail, but it is expected that you have a base understanding of this process from your previous courses, focussing on the big picture.
The pancreas is a glandular organ located in the abdomen. It plays a critical role in converting the food we eat into fuel for our bodies. In terms of functionality, the pancreas can be broken down into two main parts the exocrine pancreas which aids in digestion and the endocrine pancreas which regulates blood sugar.
The bulk of the pancreas is composed of exocrine cells which produce enzymes that aid in digestion. When food enters the stomach, the exocrine cells release their digestive enzymes into a series of small ducts that eventually join together into the main pancreatic duct.
The pancreatic duct runs the length of the pancreas and releases the digestive enzymes along with other secretions, collectively called pancreatic juice, into the small intestine.
The second functional component of the pancreas is the endocrine pancreas. The endocrine pancreas is composed of small islands of cells called the islets of Langerhans. There are at least 4 cell types found within the islets which produce hormones that are released into the blood stream and help regulate blood glucose levels.
This information is summarized in table 1. The functional distribution of the four cell types within the islets of Langerhans is shown in figure 2. Beta cells, which make up the majority of the islets, are located centrally and surrounded by the alpha, delta and F cells.
This learning object is above the course level and for your information only. This learning object is beyond what you are expected to know; however, think about where the cell type subsets are situated and the functional role location might have.
Glucagon and insulin are antagonistic hormones and somatostatin inhibits them both! It makes sense that they are all found close together. Before pancreatic hormones make it into circulation and act on tissues around the body, they first play a role in paracrine regulation of the pancreas itself.
This paracrine feedback system is demonstrated in figure 3. Take note of the signalling contrast between glucagon and insulin and somatostatin. Glucagon will always stimulate the release of the other two hormones, while insulin and somatostatin both have an inhibitory effect on the other hormones in this relationship.
Insulin and glucagon have opposing actions on one another, so if you learn one, you know the other! And remember that somatostatin will always inhibit both insulin and glucagon release. Secretion of insulin, from beta cells within the islet of Langerhans, inhibits the surrounding alpha cells from releasing glucagon and the delta cells from releasing somatostatin.
Secretion of glucagon, which is antagonistic to insulin, stimulates the delta cells to release somatostatin. Interestingly, glucagon also activates the beta cells and stimulates insulin release.
Considering insulin and glucagon have opposing actions throughout the body this may seem counterintuitive. To better understand why this occurs imagine a runner nearing the end of a marathon — after running almost 42 kilometers, glucose within the body will be severely depleted. In order to correct this state of hypoglycaemia, alpha cells in the pancreas will release glucagon, resulting in the production and liberation of glucose from the liver and adipocytes.
Insulin is then needed to help cells around the body especially the skeletal muscle cells absorb the liberated glucose and use it for energy. This interplay between glucagon and insulin allows the runner to keep moving and finish the race! It is also important to understand that not all regulatory signals are equal; the stimulatory effects that glucagon has on beta cells is much smaller than the stimulatory effects of increased blood sugar after eating a meal.
Lastly, secretion of somatostatin within the islets inhibits the activity of both the beta and alpha cells. Regulation of pancreatic hormones is a complex process, involving much more than just the paracrine feedback system within the islet of Langerhans.
Secretion of insulin and glucagon is controlled by the integration and interaction of multiple inputs including nutrients, hormones, neurotransmitters and drugs. For both insulin and glucagon, changes in blood glucose concentrations are the primary stimuli that activates, or inhibits, their release.
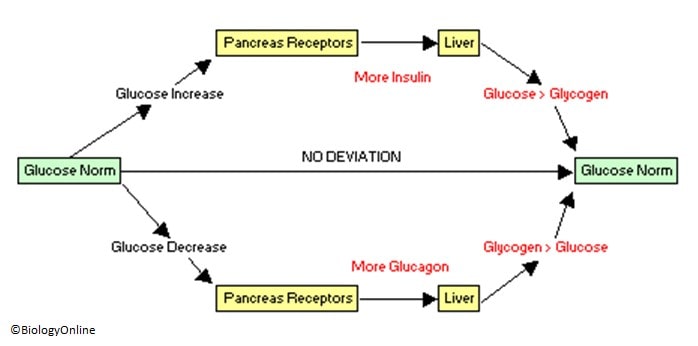
Blood glucose is the regulated variable within this system, meaning it is constantly monitored by sensors i. receptors in the body and kept within a limited range through physiological mechanisms. When the body is in a state of hyperglycemiaand blood glucose levels are elevated, sensors in the pancreas detect this and stimulate the beta cells to increase their release of insulin.
When blood glucose levels drop, putting the body is in a state of hypoglycemiathe alpha cells are stimulated and glucagon is released. The integration of blood glucose levels, and other regulatory stimuli, on alpha and beta cells is discussed in further detail below.
The following figure depicts how insulin release is regulated by different inputs throughout the body. Keep the big picture in mind. Which one of the following exhibits paracrine control?
The following figure demonstrates how the release of glucagon from alpha cells is regulated by different inputs throughout the body. Remember that both insulin and somatostatin will both inhibit the secretion of glucagon from alpha cells in the pancreas.
: Glucose regulation mechanisms| How insulin and glucagon regulate blood sugar | Physiol Rev ; 84 : — Seeley RJ, Woods SC. Monitoring of stored and available fuel by the CNS: implications for obesity. Nat Rev Neurosci ; 4 : — Woods SC, Lotter EC, McKay LD, Porte D Jr. Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Bagdade JD, Bierman EL, Porte D Jr. The significance of basal insulin levels in the evaluation of the insulin response to glucose in diabetic and nondiabetic subjects. J Clin Invest ; 46 : — Air EL, Benoit SC, Blake Smith KA, Clegg DJ, Woods SC. Acute third ventricular administration of insulin decreases food intake in two paradigms. Pharmacol Biochem Behav ; 72 : — Chavez M, Kaiyala K, Madden LJ, Schwartz MW, Woods SC. Intraventricular insulin and the level of maintained body weight in rats. Behav Neurosci ; : — Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC et al. Role of brain insulin receptor in control of body weight and reproduction. White MF. Insulin signaling in health and disease. Maffei M, Stoffel M, Barone M, Moon B, Dammerman M, Ravussin E et al. Diabetes ; 45 : — Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med ; : — Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R et al. Identification and expression cloning of a leptin receptor, OB-R. Cell ; 83 : — Coppari R, Ichinose M, Lee CE, Pullen AE, Kenny CD, McGovern RA et al. The hypothalamic arcuate nucleus: a key site for mediating leptin's effects on glucose homeostasis and locomotor activity. Cell Metab ; 1 : 63— Satoh N, Ogawa Y, Katsuura G, Hayase M, Tsuji T, Imagawa K et al. The arcuate nucleus as a primary site of satiety effect of leptin in rats. Neurosci Lett ; : — Oswal A, Yeo G. Leptin and the control of body weight: a review of its diverse central targets, signaling mechanisms, and role in the pathogenesis of obesity. Myers MG Jr, Olson DP. Central nervous system control of metabolism. Obici S, Feng Z, Morgan K, Stein D, Karkanias G, Rossetti L. Central administration of oleic acid inhibits glucose production and food intake. Diabetes ; 51 : — Miselis RR, Epstein AN. Feeding induced by intracerebroventricular 2-deoxy-D-glucose in the rat. Am J Physiol ; : — Foster DW. Malonyl-CoA: the regulator of fatty acid synthesis and oxidation. Clegg DJ, Wortman MD, Benoit SC, McOsker CC, Seeley RJ. Comparison of central and peripheral administration of C75 on food intake, body weight, and conditioned taste aversion. Obici S, Feng Z, Arduini A, Conti R, Rossetti L. Inhibition of hypothalamic carnitine palmitoyltransferase-1 decreases food intake and glucose production. Nat Med ; 9 : — Kennedy GC. The role of depot fat in the hypothalamic control of food intake in the rat. Proc R Soc Lond B Biol Sci ; : — Baggio LL, Drucker DJ. Glucagon-like peptide-1 receptors in the brain: controlling food intake and body weight. Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. Chelikani PK, Haver AC, Reidelberger RD. Intravenous infusion of glucagon-like peptide-1 potently inhibits food intake, sham feeding, and gastric emptying in rats. Am J Physiol Regul Integr Comp Physiol ; : R—R Tang-Christensen M, Larsen PJ, Goke R, Fink-Jensen A, Jessop DS, Moller M et al. Central administration of GLP amide inhibits food and water intake in rats. Am J Physiol ; : R—R Cummings DE, Clement K, Purnell JQ, Vaisse C, Foster KE, Frayo RS et al. Elevated plasma ghrelin levels in Prader Willi syndrome. Nat Med ; 8 : — Febbraio MA, Pedersen BK. Muscle-derived interleukin mechanisms for activation and possible biological roles. FASEB J ; 16 : — Wallenius V, Wallenius K, Ahren B, Rudling M, Carlsten H, Dickson SL et al. Interleukindeficient mice develop mature-onset obesity. Nat Med ; 8 : 75— Lutz TA. Control of energy homeostasis by amylin. Cell Mol Life Sci ; 69 : — Campbell JE, Drucker DJ. Nat Rev Endocrinol ; 11 : — Asakawa A, Inui A, Yuzuriha H, Ueno N, Katsuura G, Fujimiya M et al. Characterization of the effects of pancreatic polypeptide in the regulation of energy balance. Gastroenterology ; : — Woods SC, Lutz TA, Geary N, Langhans W. Pancreatic signals controlling food intake; insulin, glucagon and amylin. Philos Trans R Soc Lond B Biol Sci ; : — Bernard C. Leçons de physiologie expérimentale appliquée à la médecine. Ballière et Fils: Paris, France, Google Scholar. Anand B, Chhina G, Sharma K, Dua S, Singh B. Activity of single neurons in the hypothalamic feeding centers: effect of glucose. Oomura Y, Ono T, Ooyama H, Wayner M. Glucose and osmosensitive neurones of the rat hypothalamus. Borg MA, Sherwin RS, Borg WP, Tamborlane WV, Shulman GI. Local ventromedial hypothalamus glucose perfusion blocks counterregulation during systemic hypoglycemia in awake rats. J Clin Invest ; 99 : — Routh VH. Glucose-sensing neurons: are they physiologically relevant? Physiol Behav ; 76 : — Dunn-Meynell AA, Rawson NE, Levin BE. Brain Res ; : 41— Mizuno Y, Oomura Y. Glucose responding neurons in the nucleus tractus solitarius of the rat: in vitro study. Funahashi M, Adachi A. Glucose-responsive neurons exist within the area postrema of the rat: in vitro study on the isolated slice preparation. Brain Res Bull ; 32 : — Yettefti K, Orsini J-C, Perrin J. Characteristics of glycemia-sensitive neurons in the nucleus tractus solitarii: possible involvement in nutritional regulation. Physiol Behav ; 61 : 93— Obici S, Feng Z, Karkanias G, Baskin DG, Rossetti L. Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nat Neurosci ; 5 : — Obici S, Zhang BB, Karkanias G, Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Gelling RW, Morton GJ, Morrison CD, Niswender KD, Myers MG, Rhodes CJ et al. Insulin action in the brain contributes to glucose lowering during insulin treatment of diabetes. Cell Metab ; 3 : 67— Spanswick D, Smith M, Mirshamsi S, Routh V, Ashford M. Nat Neurosci ; 3 : — Pocai A, Lam TK, Gutierrez-Juarez R, Obici S, Schwartz GJ, Bryan J et al. Hypothalamic KATP channels control hepatic glucose production. Inoue H, Ogawa W, Asakawa A, Okamoto Y, Nishizawa A, Matsumoto M et al. Role of hepatic STAT3 in brain-insulin action on hepatic glucose production. Cell Metab ; 3 : — Coleman D. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia ; 14 : — Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T et al. Rossetti L, Massillon D, Barzilai N, Vuguin P, Chen W, Hawkins M et al. Short term effects of leptin on hepatic gluconeogenesis and in vivo insulin action. Intracerebroventricular leptin regulates hepatic but not peripheral glucose fluxes. Schwartz MW, Baskin DG, Bukowski TR, Kuijper JL, Foster D, Lasser G et al. Shimomura I, Hammer RE, Ikemoto S, Brown MS, Goldstein JL. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature ; : 73— Ebihara K, Ogawa Y, Masuzaki H, Shintani M, Miyanaga F, Aizawa-Abe M et al. Transgenic overexpression of leptin rescues insulin resistance and diabetes in a mouse model of lipoatrophic diabetes. Diabetes ; 50 : — Asilmaz E, Cohen P, Miyazaki M, Dobrzyn P, Ueki K, Fayzikhodjaeva G et al. Site and mechanism of leptin action in a rodent form of congenital lipodystrophy. Pocai A, Morgan K, Buettner C, Gutierrez-Juarez R, Obici S, Rossetti L. Central leptin acutely reverses diet-induced hepatic insulin resistance. Diabetes ; 54 : — Buettner C, Pocai A, Muse ED, Etgen AM, Myers MG, Rossetti L. Critical role of STAT3 in leptin's metabolic actions. Cell Metab ; 4 : 49— Kievit P, Howard JK, Badman MK, Balthasar N, Coppari R, Mori H et al. Enhanced leptin sensitivity and improved glucose homeostasis in mice lacking suppressor of cytokine signaling-3 in POMC-expressing cells. Cell Metab ; 4 : — Morton GJ, Gelling RW, Niswender KD, Morrison CD, Rhodes CJ, Schwartz MW. Leptin regulates insulin sensitivity via phosphatidylinositolOH kinase signaling in mediobasal hypothalamic neurons. Cell Metab ; 2 : — Borg WP, Sherwin RS, During MJ, Borg MA, Shulman GI. Local ventromedial hypothalamus glucopenia triggers counterregulatory hormone release. Diabetes ; 44 : — Ritter S, Dinh TT, Zhang Y. Localization of hindbrain glucoreceptive sites controlling food intake and blood glucose. Brain Res ; : 37— Ritter S, Bugarith K, Dinh TT. Immunotoxic destruction of distinct catecholamine subgroups produces selective impairment of glucoregulatory responses and neuronal activation. Schuit FC, Huypens P, Heimberg H, Pipeleers DG. Glucose sensing in pancreatic β-cells a model for the study of other glucose-regulated cells in gut, pancreas, and hypothalamus. Diabetes ; 50 : 1— Marty N, Dallaporta M, Thorens B. Brain glucose sensing, counterregulation, and energy homeostasis. Physiology ; 22 : — Burcelin R, Thorens B. Evidence that extrapancreatic GLUT2-dependent glucose sensors control glucagon secretion. Marty N, Dallaporta M, Foretz M, Emery M, Tarussio D, Bady I et al. Regulation of glucagon secretion by glucose transporter type 2 glut2 and astrocyte-dependent glucose sensors. Sanders NM, Dunn-Meynell AA, Levin BE. Third ventricular alloxan reversibly impairs glucose counterregulatory responses. Diabetes ; 53 : — Miki T, Liss B, Minami K, Shiuchi T, Saraya A, Kashima Y et al. Nat Neurosci ; 4 : — Evans ML, McCrimmon RJ, Flanagan DE, Keshavarz T, Fan X, McNay EC et al. McCrimmon RJ, Evans ML, Fan X, McNay EC, Chan O, Ding Y et al. Oomura Y, Ooyama H, Sugimori M, Nakamura T, Yamada Y. Glucose inhibition of the glucose-sensitive neurone in the rat lateral hypothalamus. Song Z, Levin BE, McArdle JJ, Bakhos N, Routh VH. Convergence of pre-and postsynaptic influences on glucosensing neurons in the ventromedial hypothalamic nucleus. Hardie DG, Carling D, Carlson M. Annu Rev Biochem ; 67 : — Rutter G, daSilva Xavier G, Leclerc I. Biochem J ; : 1— Kim M-S, Park J-Y, Namkoong C, Jang P-G, Ryu J-W, Song H-S et al. Anti-obesity effects of α-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase. Nat Med ; 10 : — Minokoshi Y, Alquier T, Furukawa N, Kim Y-B, Lee A, Xue B et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. McCrimmon RJ, Fan X, Ding Y, Zhu W, Jacob RJ, Sherwin RS. Potential role for AMP-activated protein kinase in hypoglycemia sensing in the ventromedial hypothalamus. McCrimmon RJ, Fan X, Cheng H, McNay E, Chan O, Shaw M et al. Activation of AMP-activated protein kinase within the ventromedial hypothalamus amplifies counterregulatory hormone responses in rats with defective counterregulation. Diabetes ; 55 : — Han S-M, Namkoong C, Jang P, Park I, Hong S, Katakami H et al. Hypothalamic AMP-activated protein kinase mediates counter-regulatory responses to hypoglycaemia in rats. Diabetologia ; 48 : — Lam TK, Pocai A, Gutierrez-Juarez R, Obici S, Bryan J, Aguilar-Bryan L et al. Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis. Nat Med ; 11 : — Okamoto H, Obici S, Accili D, Rossetti L. Restoration of liver insulin signaling in Insr knockout mice fails to normalize hepatic insulin action. Lin HV, Plum L, Ono H, Gutiérrez-Juárez R, Shanabrough M, Borok E et al. Divergent regulation of energy expenditure and hepatic glucose production by insulin receptor in agouti-related protein and POMC neurons. Diabetes ; 59 : — Ramnanan CJ, Saraswathi V, Smith MS, Donahue EP, Farmer B, Farmer TD et al. Brain insulin action augments hepatic glycogen synthesis without suppressing glucose production or gluconeogenesis in dogs. Cherrington A, Moore M, Sindelar D, Edgerton D. Insulin action on the liver in vivo. Biochem Soc Trans ; 35 : — Hendrick GK, Frizzell RT, Williams PE, Cherrington AD. Effect of hyperglucagonemia on hepatic glycogenolysis and gluconeogenesis after a prolonged fast. Am J Physiol ; : E—E Nuttall FQ, Ngo A, Gannon MC. Regulation of hepatic glucose production and the role of gluconeogenesis in humans: is the rate of gluconeogenesis constant? Diabetes Metab Res Rev ; 24 : — Kokubun E, Hirabara SM, Fiamoncini J, Curi R, Haebisch H. Changes of glycogen content in liver, skeletal muscle, and heart from fasted rats. Cell Biochem Funct ; 27 : — Shimazu T, Sudo M, Minokoshi Y, Takahashi A. Role of the hypothalamus in insulin-independent glucose uptake in peripheral tissues. Brain Res Bull ; 27 : — Sudo M, Minokoshi Y, Shimazu T. Ventromedial hypothalamic stimulation enhances peripheral glucose uptake in anesthetized rats. Minokoshi Y, Okano Y, Shimazu T. Regulatory mechanism of the ventromedial hypothalamus in enhancing glucose uptake in skeletal muscles. Minokoshi Y, Haque MS, Shimazu T. Microinjection of leptin into the ventromedial hypothalamus increases glucose uptake in peripheral tissues in rats. Diabetes ; 48 : — Roman EA, Reis D, Romanatto T, Maimoni D, Ferreira EA, Santos GA et al. Central leptin action improves skeletal muscle AKT, AMPK, and PGC1α activation by hypothalamic PI3K-dependent mechanism. Mol Cell Endocrinol ; : 62— Koch C, Augustine RA, Steger J, Ganjam GK, Benzler J, Pracht C et al. Leptin rapidly improves glucose homeostasis in obese mice by increasing hypothalamic insulin sensitivity. J Neurosci ; 30 : — Funai K, Cartee GD. Inhibition of contraction-stimulated AMP-activated protein kinase inhibits contraction-stimulated increases in PAS-TBC1D1 and glucose transport without altering PAS-AS in rat skeletal muscle. Diabetes ; 58 : — Hutchinson DS, Bengtsson T. AMP-activated protein kinase activation by adrenoceptors in L6 skeletal muscle cells mediation by α1-adrenoceptors causing glucose uptake. Minokoshi Y, Kim Y-B, Peroni OD, Fryer LG, Müller C, Carling D et al. Shiuchi T, Haque MS, Okamoto S, Inoue T, Kageyama H, Lee S et al. Hypothalamic orexin stimulates feeding-associated glucose utilization in skeletal muscle via sympathetic nervous system. Cell Metab ; 10 : — Ahrén B. Autonomic regulation of islet hormone secretion—implications for health and disease. Diabetologia ; 43 : — Article PubMed Google Scholar. Satin LS, Kinard TA. Neurotransmitters and their receptors in the islets of Langerhans of the pancreas. Endocrine ; 8 : — Thorens B. Central control of glucose homeostasis: the brain—endocrine pancreas axis. Diabetes Metab ; 36 : S45—S Ionescu E, Rohner-Jeanrenaud F, Berthoud H-R, Jeanrenaud B. Increases in plasma insulin levels in response to electrical stimulation of the dorsal motor nucleus of the vagus nerve. Chen M, Woods SC, Porte D. Effect of cerebral intraventricular insulin on pancreatic insulin secretion in the dog. Diabetes ; 24 : — Paranjape SA, Chan O, Zhu W, Horblitt AM, McNay EC, Cresswell JA et al. Influence of insulin in the ventromedial hypothalamus on pancreatic glucagon secretion in vivo. Brain glucose sensing and neural regulation of insulin and glucagon secretion. Diabetes Obes Metab ; 13 : 82— Caro JF, Kolaczynski JW, Nyce MR, Ohannesian JP, Opentanova I, Goldman WH et al. Lancet ; : — Maes HH, Neale MC, Eaves LJ. Genetic and environmental factors in relative body weight and human adiposity. Behav Genet ; 27 : — Kubota N, Terauchi Y, Tobe K, Yano W, Suzuki R, Ueki K et al. Insulin receptor substrate 2 plays a crucial role in beta cells and the hypothalamus. Gao Q, Wolfgang MJ, Neschen S, Morino K, Horvath TL, Shulman GI et al. Disruption of neural signal transducer and activator of transcription 3 causes obesity, diabetes, infertility, and thermal dysregulation. Proc Natl Acad Sci USA ; : — El-Haschimi K, Pierroz DD, Hileman SM, Bjørbæk C, Flier JS. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. Mori H, Hanada R, Hanada T, Aki D, Mashima R, Nishinakamura H et al. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Egawa K, Maegawa H, Shimizu S, Morino K, Nishio Y, Bryer-Ash M et al. Protein-tyrosine phosphatase-1B negatively regulates insulin signaling in l6 myocytes and Fao hepatoma cells. Kaszubska W, Falls HD, Schaefer VG, Haasch D, Frost L, Hessler P et al. Protein tyrosine phosphatase 1B negatively regulates leptin signaling in a hypothalamic cell line. Mol Cell Endocrinol ; : — Bence KK, Delibegovic M, Xue B, Gorgun CZ, Hotamisligil GS, Neel BG et al. Neuronal PTP1B regulates body weight, adiposity and leptin action. Nat Med ; 12 : — Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Cell ; : 61— Ozcan L, Ergin AS, Lu A, Chung J, Sarkar S, Nie D et al. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab ; 9 : 35— Ottaway N, Mahbod P, Rivero B, Norman LA, Gertler A, D'Alessio DA et al. Diet-induced obese mice retain endogenous leptin action. Cell Metab ; 21 : — Alwan A. Global Status Report on Noncommunicable Diseases World Health Organization: Geneva, Switzerland, Ono H, Pocai A, Wang Y, Sakoda H, Asano T, Backer JM et al. Activation of hypothalamic S6 kinase mediates diet-induced hepatic insulin resistance in rats. CAS PubMed PubMed Central Google Scholar. Download references. This work was supported by grants from the National Research Foundation NRFR1A6A3A, NRFM3C7A for M-SK and the Asan Institute for Life Sciences Appeptite Regulation Laboratory, Asan Institute for Life Sciences, University of Ulsan College of Medicine, Seoul, Korea. Department of Medicine, University of Ulsan College of Medicine, Seoul, Korea. Division of Endocrinology and Metabolism, Asan Medical Center, Seoul, Korea. You can also search for this author in PubMed Google Scholar. Correspondence to Min-Seon Kim. This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4. Reprints and permissions. Roh, E. Emerging role of the brain in the homeostatic regulation of energy and glucose metabolism. Exp Mol Med 48 , e Download citation. Received : 20 November Revised : 07 December Accepted : 09 December Published : 11 March Issue Date : March Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Molecular and Cellular Biochemistry Skip to main content Thank you for visiting nature. Download PDF. Subjects Endocrinology Medical research. Abstract Accumulated evidence from genetic animal models suggests that the brain, particularly the hypothalamus, has a key role in the homeostatic regulation of energy and glucose metabolism. Central regulation of energy metabolism In normal individuals, food intake and energy expenditure are tightly regulated by homeostatic mechanisms to maintain energy balance. Full size image. Brain regulation of glucose metabolism The earliest demonstration of the role of the brain in glucose homeostasis was provided by the physiologist Claude Bernard in Figure 2. Figure 3. Concluding remarks This review highlights the role of the brain in the homeostatic regulation of energy and glucose metabolism. References Morton GJ, Meek TH, Schwartz MW. Article CAS PubMed PubMed Central Google Scholar Sandoval D, Cota D, Seeley RJ. Article CAS PubMed Google Scholar Schwartz MW, Porte D Jr. Article CAS PubMed Google Scholar Broadwell RD, Brightman MW. Article CAS PubMed Google Scholar Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG. Article CAS PubMed Google Scholar Heisler LK, Cowley MA, Tecott LH, Fan W, Low MJ, Smart JL et al. Article CAS PubMed Google Scholar Bouret SG, Draper SJ, Simerly RB. Article CAS PubMed PubMed Central Google Scholar Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR et al. Article CAS PubMed Google Scholar Tao YX. Article CAS PubMed Google Scholar Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I et al. Article CAS PubMed Google Scholar Bewick GA, Gardiner JV, Dhillo WS, Kent AS, White NE, Webster Z et al. Article CAS PubMed Google Scholar Yulyaningsih E, Zhang L, Herzog H, Sainsbury A. Article CAS PubMed PubMed Central Google Scholar Bingham NC, Anderson KK, Reuter AL, Stallings NR, Parker KL. Article CAS PubMed PubMed Central Google Scholar Foster MT, Song CK, Bartness TJ. Article Google Scholar Leibowitz SF, Hammer NJ, Chang K. Article CAS PubMed Google Scholar Gonzalez JA, Reimann F, Burdakov D. Article CAS PubMed Google Scholar Fei H, Okano HJ, Li C, Lee GH, Zhao C, Darnell R et al. Article CAS PubMed PubMed Central Google Scholar Xu B, Goulding EH, Zang K, Cepoi D, Cone RD, Jones KR et al. Article CAS PubMed PubMed Central Google Scholar Shimizu N, Oomura Y, Plata-Salaman CR, Morimoto M. Article CAS PubMed Google Scholar Jacobowitz DM, O'Donohue TL. Article CAS PubMed PubMed Central Google Scholar Bernardis LL, Bellinger LL. Article CAS PubMed Google Scholar Broberger C, De Lecea L, Sutcliffe JG, Hokfelt T. Article CAS PubMed Google Scholar Ohno K, Sakurai T. Article CAS PubMed Google Scholar Hungs M, Mignot E. Article CAS PubMed Google Scholar Marsh DJ, Weingarth DT, Novi DE, Chen HY, Trumbauer ME, Chen AS et al. Article CAS PubMed PubMed Central Google Scholar Schwartz GJ. Article CAS PubMed Google Scholar Stanley S, Wynne K, McGowan B, Bloom S. Article CAS PubMed Google Scholar Geerling JC, Shin JW, Chimenti PC, Loewy AD. Article PubMed PubMed Central Google Scholar Ahima RS, Antwi DA. Article CAS PubMed PubMed Central Google Scholar Ellacott KL, Halatchev IG, Cone RD. Article CAS PubMed Google Scholar Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB et al. Article CAS PubMed Google Scholar Kim KS, Yoon YR, Lee HJ, Yoon S, Kim SY, Shin SW et al. Article CAS PubMed PubMed Central Google Scholar Spiegelman BM, Flier JS. Article CAS PubMed Google Scholar Kramer A, Yang FC, Snodgrass P, Li X, Scammell TE, Davis FC et al. Article CAS PubMed Google Scholar Samson WK, Bagley SL, Ferguson AV, White MM. Article CAS Google Scholar Nakamachi T, Matsuda K, Maruyama K, Miura T, Uchiyama M, Funahashi H et al. Article CAS PubMed Google Scholar Huo L, Gamber K, Greeley S, Silva J, Huntoon N, Leng XH et al. Article CAS PubMed PubMed Central Google Scholar Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D et al. Article CAS PubMed Google Scholar Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J et al. Article CAS PubMed Google Scholar Dodd GT, Decherf S, Loh K, Simonds SE, Wiede F, Balland E et al. Article CAS PubMed PubMed Central Google Scholar Morrison SF, Madden CJ, Tupone D. Article CAS PubMed PubMed Central Google Scholar Sacks H, Symonds ME. Article CAS PubMed PubMed Central Google Scholar Ouellet V, Routhier-Labadie A, Bellemare W, Lakhal-Chaieb L, Turcotte E, Carpentier AC et al. Article CAS PubMed Google Scholar Seoane-Collazo P, Ferno J, Gonzalez F, Dieguez C, Leis R, Nogueiras R et al. Article CAS PubMed Google Scholar Imai-Matsumura K, Matsumura K, Nakayama T. Article CAS PubMed Google Scholar Yoshida K, Li X, Cano G, Lazarus M, Saper CB. Article CAS PubMed PubMed Central Google Scholar Zhang Y, Kerman IA, Laque A, Nguyen P, Faouzi M, Louis GW et al. Article CAS PubMed PubMed Central Google Scholar Chao PT, Yang L, Aja S, Moran TH, Bi S. Article CAS PubMed PubMed Central Google Scholar Brito MN, Brito NA, Baro DJ, Song CK, Bartness TJ. Article CAS PubMed Google Scholar Haynes WG, Morgan DA, Djalali A, Sivitz WI, Mark AL. Article CAS PubMed Google Scholar Lockie SH, Heppner KM, Chaudhary N, Chabenne JR, Morgan DA, Veyrat-Durebex C et al. Article CAS PubMed PubMed Central Google Scholar Rahmouni K, Morgan DA, Morgan GM, Liu X, Sigmund CD, Mark AL et al. Article CAS PubMed PubMed Central Google Scholar Rothwell NJ, Stock MJ. Article CAS PubMed Google Scholar Schwartz RS, Jaeger LF, Veith RC. CAS PubMed Google Scholar Cannon B, Nedergaard J. Article CAS PubMed Google Scholar Seeley RJ, Woods SC. Article CAS PubMed Google Scholar Woods SC, Lotter EC, McKay LD, Porte D Jr. Article CAS PubMed Google Scholar Bagdade JD, Bierman EL, Porte D Jr. Article CAS PubMed PubMed Central Google Scholar Air EL, Benoit SC, Blake Smith KA, Clegg DJ, Woods SC. Article CAS PubMed Google Scholar Chavez M, Kaiyala K, Madden LJ, Schwartz MW, Woods SC. Article CAS PubMed Google Scholar Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC et al. Article CAS PubMed Google Scholar White MF. Article CAS PubMed Google Scholar Maffei M, Stoffel M, Barone M, Moon B, Dammerman M, Ravussin E et al. Article CAS PubMed Google Scholar Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR et al. Article CAS PubMed Google Scholar Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R et al. Article CAS PubMed Google Scholar Coppari R, Ichinose M, Lee CE, Pullen AE, Kenny CD, McGovern RA et al. Article CAS PubMed Google Scholar Satoh N, Ogawa Y, Katsuura G, Hayase M, Tsuji T, Imagawa K et al. Article CAS PubMed Google Scholar Oswal A, Yeo G. Article Google Scholar Myers MG Jr, Olson DP. Article CAS PubMed Google Scholar Obici S, Feng Z, Morgan K, Stein D, Karkanias G, Rossetti L. Article CAS PubMed Google Scholar Miselis RR, Epstein AN. Article CAS PubMed Google Scholar Foster DW. Article CAS PubMed PubMed Central Google Scholar Clegg DJ, Wortman MD, Benoit SC, McOsker CC, Seeley RJ. Article CAS PubMed Google Scholar Obici S, Feng Z, Arduini A, Conti R, Rossetti L. Article CAS PubMed Google Scholar Kennedy GC. Article CAS PubMed Google Scholar Baggio LL, Drucker DJ. Article CAS PubMed PubMed Central Google Scholar Merchenthaler I, Lane M, Shughrue P. Article CAS PubMed Google Scholar Chelikani PK, Haver AC, Reidelberger RD. Article CAS PubMed Google Scholar Tang-Christensen M, Larsen PJ, Goke R, Fink-Jensen A, Jessop DS, Moller M et al. CAS PubMed Google Scholar Cummings DE, Clement K, Purnell JQ, Vaisse C, Foster KE, Frayo RS et al. Article CAS PubMed Google Scholar Febbraio MA, Pedersen BK. Article CAS PubMed Google Scholar Wallenius V, Wallenius K, Ahren B, Rudling M, Carlsten H, Dickson SL et al. Article CAS PubMed Google Scholar Lutz TA. Article CAS PubMed Google Scholar Campbell JE, Drucker DJ. Article CAS PubMed Google Scholar Asakawa A, Inui A, Yuzuriha H, Ueno N, Katsuura G, Fujimiya M et al. Article CAS PubMed Google Scholar Woods SC, Lutz TA, Geary N, Langhans W. Thorens B. Diabetes Obes Metab , 13 Suppl , 01 Oct Cited by: articles PMID: Abdulla H , Phillips B , Smith K , Wilkinson D , Atherton PJ , Idris I. Curr Diabetes Rev , 10 5 , 01 Jan Cited by: 14 articles PMID: Contact us. Europe PMC requires Javascript to function effectively. Recent Activity. Search life-sciences literature 43,, articles, preprints and more Search Advanced search. This website requires cookies, and the limited processing of your personal data in order to function. By using the site you are agreeing to this as outlined in our privacy notice and cookie policy. Triplitt CL 1. Affiliations 1. Department of Medicine, Division of Diabetes, University of Texas Health Science Center at San Antonio, TX, USA. Authors Triplitt CL 1. Share this article Share with email Share with twitter Share with linkedin Share with facebook. Macrovascular and microvascular complications can occur; DM is a major cause of heart disease and stroke, and is the seventh leading cause of death in the United States. Newly elucidated mechanisms include the involvement of the kidneys in glucose regulation, as well as central glucose regulation by the brain. This article provides an extensive review of mechanisms involved in physiologic blood glucose regulation and imbalances in glucose homeostasis. Is Environmental Cadmium Exposure Causally Related to Diabetes and Obesity? Satarug S Cells , 13 1 , 30 Dec Cited by: 0 articles PMID: PMCID: PMC Review Articles in the Open Access Subset are available under a Creative Commons license. Comprehensive Approach to Medical Nutrition Therapy in Patients with Type 2 Diabetes Mellitus: From Diet to Bioactive Compounds. Barrea L , Vetrani C , Verde L , Frias-Toral E , Ceriani F , Cernea S , Docimo A , Graziadio C , Tripathy D , Savastano S , Colao A , Muscogiuri G Antioxidants Basel , 12 4 , 10 Apr Cited by: 3 articles PMID: PMCID: PMC Review Articles in the Open Access Subset are available under a Creative Commons license. Pancreatic β-cell glutaminase 2 maintains glucose homeostasis under the condition of hyperglycaemia. Deguchi-Horiuchi H , Suzuki S , Lee EY , Miki T , Yamanaka N , Manabe I , Tanaka T , Yokote K Sci Rep , 13 1 , 05 May Cited by: 0 articles PMID: PMCID: PMC Articles in the Open Access Subset are available under a Creative Commons license. The association between glycemic index and glycemic load and quality of life among overweight and obese women: a cross-sectional study. Rasaei N , Fallah M , Gholami F , Karimi M , Noori S , Bahrampour N , Clark CCT , Mirzaei K BMC Nutr , 9 1 , 13 Feb Cited by: 0 articles PMID: PMCID: PMC Articles in the Open Access Subset are available under a Creative Commons license. Metabolism and Health Impacts of Dietary Sugars. Alam YH , Kim R , Jang C J Lipid Atheroscler , 11 1 , 17 Jan Cited by: 8 articles PMID: PMCID: PMC Review Articles in the Open Access Subset are available under a Creative Commons license. Similar Articles To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation. Impaired incretin secretion and pancreatic dysfunction with older age and diabetes. Geloneze B , de Oliveira Mda S , Vasques AC , Novaes FS , Pareja JC , Tambascia MA Metabolism , 63 7 , 12 Apr Cited by: 16 articles PMID: Genetic determinants of glucose homeostasis. Barker A , Langenberg C , Wareham NJ Best Pract Res Clin Endocrinol Metab , 26 2 , 01 Apr Cited by: 8 articles PMID: Review. The pathophysiologic defects of type 2 diabetes. Abnormal insulin action and impaired insulin secretion. Kahn SE Postgrad Med , 6 suppl key , 01 May Cited by: 0 articles PMID: Review. Brain glucose sensing and neural regulation of insulin and glucagon secretion. Thorens B Diabetes Obes Metab , 13 Suppl , 01 Oct Cited by: articles PMID: Review. Physiological mechanisms of action of incretin and insulin in regulating skeletal muscle metabolism. Abdulla H , Phillips B , Smith K , Wilkinson D , Atherton PJ , Idris I Curr Diabetes Rev , 10 5 , 01 Jan Cited by: 14 articles PMID: Review. Claim to ORCID Get citation. Follow us News blog Technical blog Twitter YouTube. About About Europe PMC. Become a funder. Tools Tools overview. ORCID article claiming. Journal list. Grant finder. External links service. |
| Examining the Mechanisms of Glucose Regulation | Storage of fat. How would you explain the function of insulin to your patient with diabetes? What does it turn on and what does it turn off? Glucagon , a peptide hormone secreted by the pancreas, raises blood glucose levels. Its effect is opposite to insulin, which lowers blood glucose levels. When it reaches the liver, glucagon stimulates glycolysis , the breakdown of glycogen, and the export of glucose into the circulation. The pancreas releases glucagon when glucose levels fall too low. Glucagon causes the liver to convert stored glycogen into glucose, which is released into the bloodstream. High BG levels stimulate the release of insulin. Insulin allows glucose to be taken up and used by insulin-dependent tissues, such as muscle cells. Glucagon and insulin work together automatically as a negative feedback system to keeps BG levels stable. Glucagon is a powerful regulator of BG levels, and glucagon injections can be used to correct severe hypoglycemia. Glucose taken orally or parenterally can elevate plasma glucose levels within minutes, but exogenous glucagon injections are not glucose; a glucagon injection takes approximately 10 to 20 minutes to be absorbed by muscle cells into the bloodstream and circulated to the liver, there to trigger the breakdown of stored glycogen. People with type 2 diabetes have excess glucagon secretion, which is a contributor to the chronic hyperglycemia of type 2 diabetes. The amazing balance of these two opposing hormones of glucagon and insulin is maintained by another pancreatic hormone called somatostatin , created in the delta cells. It truly is the great pancreatic policeman as it works to keep them balanced. When it goes too high the pancreas releases insulin into the bloodstream. This insulin stimulates the liver to convert the blood glucose into glycogen for storage. If the blood sugar goes too low, the pancreas release glucagon, which causes the liver to turn stored glycogen back into glucose and release it into the blood. Source: Google Images. Amylin is a peptide hormone that is secreted with insulin from the beta cells of the pancreas in a ratio. Amylin inhibits glucagon secretion and therefore helps lower BG levels. It also delays gastric emptying after a meal to decrease a sudden spike in plasma BG levels; further, it increases brain satiety satisfaction to help someone feel full after a meal. This is a powerful hormone in what has been called the brain—meal connection. People with type 1 diabetes have neither insulin nor amylin production. People with type 2 diabetes seem to make adequate amounts of amylin but often have problems with the intestinal incretin hormones that also regulate BG and satiety, causing them to feel hungry constantly. Amylin analogues have been created and are available through various pharmaceutical companies as a solution for disorders of this hormone. Incretins go to work even before blood glucose levels rise following a meal. They also slow the rate of absorption of nutrients into the bloodstream by reducing gastric emptying, and they may also help decrease food intake by increasing satiety. People with type 2 diabetes have lower than normal levels of incretins, which may partly explain why many people with diabetes state they constantly feel hungry. After research showed that BG levels are influenced by intestinal hormones in addition to insulin and glucagon, incretin mimetics became a new class of medications to help balance BG levels in people who have diabetes. Two types of incretin hormones are GLP-1 glucagon-like peptide and GIP gastric inhibitory polypeptide. Each peptide is broken down by naturally occurring enzymes called DDP-4, dipeptidyl peptidase Exenatide Byetta , an injectable anti-diabetes drug, is categorized as a glucagon-like peptide GLP-1 and directly mimics the glucose-lowering effects of natural incretins upon oral ingestion of carbohydrates. The administration of exenatide helps to reduce BG levels by mimicking the incretins. Both long- and short-acting forms of GLP-1 agents are currently being used. A new class of medications, called DPP4 inhibitors, block this enzyme from breaking down incretins, thereby prolonging the positive incretin effects of glucose suppression. An additional class of medications called dipeptidyl peptidase-4 DPP-4 inhibitors—note hyphen , are available in the form of several orally administered products. These agents will be discussed more fully later. People with diabetes have frequent and persistent hyperglycemia, which is the hallmark sign of diabetes. For people with type 1 diabetes, who make no insulin, glucose remains in the blood plasma without the needed BG-lowering effect of insulin. Another contributor to this chronic hyperglycemia is the liver. When a person with diabetes is fasting, the liver secretes too much glucose, and it continues to secrete glucose even after the blood level reaches a normal range Basu et al. Another contributor to chronic hyperglycemia in diabetes is skeletal muscle. After a meal, the muscles in a person with diabetes take up too little glucose, leaving blood glucose levels elevated for extended periods Basu et al. The metabolic malfunctioning of the liver and skeletal muscles in type 2 diabetes results from a combination of insulin resistance, beta cell dysfunction, excess glucagon, and decreased incretins. These problems develop progressively. Early in the disease the existing insulin resistance can be counteracted by excess insulin secretion from the beta cells of the pancreas, which try to address the hyperglycemia. The hyperglycemia caused by insulin resistance is met by hyperinsulinemia. Eventually, however, the beta cells begin to fail. Hyperglycemia can no longer be matched by excess insulin secretion, and the person develops clinical diabetes Maitra, How would you explain to your patient what lifestyle behaviors create insulin resistance? In type 2 diabetes, many patients have body cells with a decreased response to insulin known as insulin resistance. This means that, for the same amount of circulating insulin, the skeletal muscles, liver, and adipose tissue take up and metabolize less glucose than normal. Insulin resistance can develop in a person over many years before the appearance of type 2 diabetes. People inherit a propensity for developing insulin resistance, and other health problems can worsen the condition. For example, when skeletal muscle cells are bathed in excess free fatty acids, the cells preferentially use the fat for metabolism while taking up and using less glucose than normal, even when there is plenty of insulin available. In this way, high levels of blood lipids decrease the effectiveness of insulin; thus, high cholesterol and body fat, overweight and obesity increase insulin resistance. Physical inactivity has a similar effect. Sedentary overweight and obese people accumulate triglycerides in their muscle cells. This causes the cells to use fat rather than glucose to produce muscular energy. Physical inactivity and obesity increase insulin resistance Monnier et al. For people with type 1 diabetes, no insulin is produced due to beta cells destruction. Triggers of that autoimmune response have been linked to milk, vaccines, environmental triggers, viruses, and bacteria. For people with type 2 diabetes, a progressive decrease in the concentration of insulin in the blood develops. Not only do the beta cells release less insulin as type 2 diabetes progresses, they also release it slowly and in a different pattern than that of healthy people Monnier et al. Without sufficient insulin, the glucose-absorbing tissues—mainly skeletal muscle, liver, and adipose tissue—do not efficiently clear excess glucose from the bloodstream, and the person suffers the damaging effects of toxic chronic hyperglycemia. At first, the beta cells manage to manufacture and release sufficient insulin to compensate for the higher demands caused by insulin resistance. Eventually, however, the defective beta cells decrease their insulin production and can no longer meet the increased demand. At this point, the person has persistent hyperglycemia. A downward spiral follows. The hyperglycemia and hyperinsulinemia caused by the over-stressed beta cells create their own failure. In type 2 diabetes, the continual loss of functioning beta cells shows up as a progressive hyperglycemia. How would you explain insulin resistance differently to someone with type 1 diabetes and someone with type 2 diabetes? Together, insulin resistance and decreased insulin secretion lead to hyperglycemia, which causes most of the health problems in diabetes. The acute health problems—diabetic ketoacidosis and hyperosmolar hyperglycemic state—are metabolic disorders that are directly caused by an overload of glucose. In comparison, the chronic health problems—eye, heart, kidney, nerve, and wound problems—are tissue injury, a slow and progressive cellular damage caused by feeding tissues too much glucose ADA, Hyperglycemic damage to tissues is the result of glucose toxicity. There are at least three distinct routes by which excess glucose injures tissues:. If you are attending a virtual event or viewing video content, you must meet the minimum participation requirement to proceed. If you think this message was received in error, please contact an administrator. You are here Home » Diabetes Type 2: Nothing Sweet About It. Diabetes Type 2: Nothing Sweet About It Course Content. Return to Course Home. Diabetes Type 2: Nothing Sweet About It Page 6 of Fuels of the Body To appreciate the pathology of diabetes, it is important to understand how the body normally uses food for energy. Hormones of the Pancreas Regulation of blood glucose is largely done through the endocrine hormones of the pancreas, a beautiful balance of hormones achieved through a negative feedback loop. The glucose becomes syrupy in the bloodstream, intoxicating cells and competing with life-giving oxygen. Glycogen phosphorylase is active when it is phosphorylated at its serine 14 residue. The phosphorylation of glycogen phosphorylase requires a cascade mechanism of epinephrine and glucagon in the liver. On the activation of Gαs by the binding of hormones to cell surface G protein-coupled receptors beta adrenergic receptor or glucagon receptor , the intracellular cyclic AMP cAMP levels increase via adenylate cyclase, leading to the activation of PKA. PKA is then responsible for the phosphorylation and activation of glycogen phosphorylase kinase, which in turn phosphorylates and activates glycogen phosphorylase to enhance glycogen breakdown. Under feeding conditions, this kinase cascade is inactive due to the lack of secretion of catabolic hormones. In addition, insulin promotes the activation of PP1, which dephosphorylates and inactivates glycogen phosphorylase. In essence, the anabolic hormone insulin promotes glycogenesis and inhibits glycogenolysis via the activation of PP1, leading to the dephosphorylation of glycogen phosphorylase inactivation and glycogen synthase activation , and via the activation of Akt, leading to the phosphorylation of GSK-3 inactivation that is unable to phosphorylate and inactivate glycogen synthase. As stated above, glycolysis is critical to the catabolism of glucose in most cells to generate energy. The key rate-limiting enzymes for this pathway include glucokinase GK, also termed hexokinase IV , which converts glucose into glucose 6-phosphate; phosphofructokinase-1 PFK-1 , which converts fructose 6-bisphosphate into fructose 1,6-bisphosphate; and liver-type pyruvate kinase L-PK , which converts phosphoenolpyruvate PEP into pyruvate in the liver. These enzymes are tightly regulated by allosteric mediators that generally promote the catabolism of glucose in the cell. GK is a high Km hexokinase that is present in the liver and the pancreatic beta cells, thus functioning as a glucose sensor for each cell type. Unlike the other hexokinase isotypes, GK activity is not allosterically inhibited by its catalytic product, glucose 6-phosphate in the cell, thus enabling the liver to continuously utilize glucose for glycolysis during conditions of increased glucose availability, such as during feeding conditions. GK is regulated via its interaction with glucokinase regulatory protein GKRP. In the low intracellular glucose concentration during fasting, the binding of GK and GKRP is enhanced by fructose 6-phosphate, leading to the nuclear localization of this protein complex. Higher concentrations of glucose during feeding compete with fructose 6-phosphate to bind this complex, which promotes the cytosolic localization of GK that is released from GKRP, thus causing the increased production of glucose 6-phosphate in this setting. PFK-1 catalyzes the metabolically irreversible step that essentially commits glucose to glycolysis. This enzyme activity is allosterically inhibited by ATP and citrate, which generally indicate a sign of energy abundance. Reciprocally, it is allosterically activated by ADP or AMP, making it more efficient to bring about glycolysis to produce more ATP in the cell. In addition, PFK-1 activity is allosterically activated by fructose 2,6-bisphosphate F26BP , a non-glycolytic metabolite that is critical for the regulation of glucose metabolism in the liver. F26BP is generated from fructose 6-phosphate by the kinase portion of a bifunctional enzyme that contains both a kinase domain phosphofructokinase-2, PFK-2 and a phosphatase domain fructose 2,6-bisphosphatase, F-2,6-Pase. PFK-2 is activated by the insulin-dependent dephosphorylation of a bifunctional enzyme that activates PFK-2 activity and simultaneously inhibits F-2,6-Pase activity to promote the increased F26BP concentration. Glucagon-mediated activation of PKA is shown to be responsible for the phosphorylation and inactivation of the kinase portion of this enzyme. Unlike its muscle counterpart, L-PK is also a critical regulatory step in the control of glycolysis in the liver. As in the case of other glycolytic enzymes, L-PK activity is regulated by both allosteric mediators and post-translational modifications. L-PK activity is allosterically activated by fructose 1,6-bisphosphate, an indicator for the active glycolysis. By contrast, its activity is allosterically inhibited by ATP, acetyl-CoA, and long-chain fatty acids, all of which signal an abundant energy supply. Additionally, the amino acid alanine inhibits its activity, as it can be readily converted to pyruvate by a transamination reaction. L-PK is inhibited by PKA following a glucagon-mediated increase in intracellular cAMP during fasting and is activated by insulin-mediated dephosphorylation under feeding conditions. In addition to the acute regulation of key regulatory enzymes, glycolysis is regulated by a transcriptional mechanism that is activated during feeding conditions. Two major transcription factors, sterol regulatory element binding protein 1c SREBP-1c and carbohydrate response element binding protein ChREBP , are responsible for the transcriptional activation of not only glycolytic enzyme genes but also the genes involved in fatty acid biosynthesis such as fatty acid synthase FAS , acetyl-CoA carboxylase ACC , and stearoyl-CoA desaturase 1 SCD1 and triacylglycerol formation such as glycerol 3-phosphate acyltransferase GPAT and diacylglycerol acyltransferase 2 DGAT2 , a process that is normally activated by a carbohydrate-rich diet Figure 2. Regulation of hepatic glycolysis. Under feeding conditions, increased glucose uptake in hepatocytes promotes glycolysis and lipogenesis to generate triglycerides as storage forms of fuel. This process is transcriptionally regulated by two major transcription factors in the liver, SREBP-1c and ChREBP-Mlx heterodimer, which mediate the insulin and glucose response, respectively. SREBPs are the major regulators of lipid metabolism in mammals. SREBP is translated as an endoplasmic reticulum ER -bound precursor form that contains the N-terminal transcription factor domain and the C-terminal regulatory domain linked with the central transmembrane domain. SREBP-1c, however, activates the genes encoding the enzymes for lipogenesis FAS, ACC, SCD1, and DGAT2 as well as GK, which is a first enzyme in the commitment step of glucose utilization in the liver. Indeed, liver-specific SREBP-1c knockout mice showed an impaired activation of lipogenic genes in a high carbohydrate diet, thus confirming the importance of this transcription factor in the regulation of hepatic glycolysis and fatty acid biosynthesis. The expression of SREBP-2 is not controlled by sterols, but its proteolytic processing is tightly regulated by intracellular concentrations of cholesterol. The exact transcription factor that mediates this insulin-dependent signal is not yet clear, although SREBP-1c itself might be involved in the process as part of an auto-regulatory loop. Interestingly, the oxysterol-sensing transcription factor liver X receptor LXR is shown to control the transcription of SREBP-1c, suggesting that SREBP-1c and SREBP-2 could be regulated differently in response to cellular cholesterol levels. In HepG2 cells, PKA was shown to reduce the DNA binding ability of SREBP-1a by the phosphorylation of serine equivalent of serine for SREBP-1c. The other prominent transcription factor for controlling glycolysis and fatty acid biosynthesis in the liver is ChREBP. ChREBP was initially known as Williams-Beuren syndrome critical region 14 WBSCR14 and was considered one of the potential genes that instigate Williams-Beuren syndrome. Later, by using a carbohydrate response element ChoRE from L-PK, ChREBP was isolated as a bona fide transcription factor for binding ChoRE of glycolytic promoters. A recent report indeed suggested a role for LXR in the transcriptional activation of ChREBP in response to glucose, although the study needs to be further verified because the transcriptional response is shown not only by the treatment of D-glucose, a natural form of glucose present in animals, but also by the treatment of unnatural L-glucose, a form of glucose that is not known to activate lipogenesis in the liver. PKA is shown to phosphorylate serine , which is critical for cellular localization, and threonine , which is critical for its DNA binding ability, whereas AMPK phosphorylate serine dictates its DNA binding ability. All three sites are phosphorylated under fasting conditions by these kinases and are dephosphorylated under feeding by xylulose 5-phosphate X5P -mediated activity of protein phosphatase 2A PP2A. First, high glucose concentrations in primary hepatocytes do not result in decreased cAMP levels or PKA activity, suggesting that other signals might be necessary to mediate the high glucose-dependent nuclear translocation of ChREBP. ChREBP knockout mice were born in a Mendelian ratio and showed no developmental problems. The knockout animals showed reduced liver triacylglycerol levels together with a reduction in lipogenic gene expression, thus confirming the role of ChREBP in the control of hepatic glycolysis and fatty acid synthesis. Prolonged fasting or starvation induces de novo glucose synthesis from non-carbohydrate precursors, termed hepatic gluconeogenesis. This process initiates from the conversion of pyruvate to oxaloacetate by pyruvate carboxylase PC in the mitochondria and eventually concludes in the conversion into glucose via several enzymatic processes in the cytosol. Key regulatory enzymes in that pathway, including glucose 6-phosphatase G6Pase , fructose 1,6-bisphosphatase Fbpase1 , PC, and phosphoenolpyruvate carboxykinase PEPCK , are activated under fasting conditions to enhance gluconeogenic flux in that setting. Mitochondrial acetyl-CoA derived from the increased fatty acid oxidation under fasting functions as a key allosteric activator of PC, leading to the increased production of oxaloacetate for the gluconeogenesis. In addition, F26BP, which is a key allosteric regulator for glycolysis by activating PFK-1, was shown to inhibit gluconeogenesis via the allosteric inhibition of Fbpase1, which helps reciprocally control gluconeogenesis and glycolysis under different dietary statuses. Because Fbpase2 is activated but PFK-2 is inhibited under fasting, the lack of F26BP enables the activation of Fbpase1 and the increased production of fructose 6-phosphate in gluconeogenesis. The chronic activation of gluconeogenesis is ultimately achieved via transcriptional mechanisms. Major transcriptional factors that are shown to induce gluconeogenic genes include CREB, FoxO1, and several nuclear receptors Figure 3. Regulation of hepatic gluconeogenesis. Under fasting conditions, hepatic gluconeogenesis is enhanced via a decreased concentration of insulin and an increased concentration of insulin counterregulatory hormones such as glucagon. FoxO1, forkhead box O 1. Under fasting conditions, glucagon and epinephrine can increase the cAMP concentration in the liver via the activation of adenylate cyclase, leading to the activation of PKA and the subsequent induction of CREB via its serine phosphorylation. In contrast, the role for CBP in gluconeogenesis is still controversial. Disruption of CREB-CBP interaction does not appear to affect glucose homeostasis because mice exhibiting a stable expression of mutant CBP that was unable to bind CREB showed normal glycemia. The CRTC family of transcriptional coactivators consists of CRTC1, CRTC2 and CRTC3, which were isolated by the expression library screening as activaters of CREB-dependent transcription. Recent studies have delineated the role of CRTC2 in the regulation of hepatic gluconeogenesis in vivo. Knockdown of CRTC2 in mice by RNAi reduced blood glucose levels and led to a concomitant repression of gluconeogenic gene expression. The forkhead box O FoxOs belongs to a class of forkhead families of transcription, which recognize the AT-rich insulin response element on the promoter. Peroxisome proliferator-activated receptor gamma coactivator 1 alpha PGC-1α , a known coactivator for nuclear receptors, functions as a key transcriptional coactivator for FoxO1 in hepatic gluconeogenesis. In this case, PRMT1 promotes the asymmetric dimethylation of arginine and in FoxO1, which blocks the binding of Akt and the subsequent Akt-mediated phosphorylation of the adjacent serine residue serine , thus enhancing the nuclear localization of FoxO1. Nuclear receptors belong to the superfamily of transcription factors that possess two Cys2-His2 type zinc finger motifs as a DNA binding domain as well as both ligand-independent and ligand-dependent transactivation domains. Nuclear receptors can be classified into one of three subgroups based on their dimer-forming potential. Homodimeric nuclear receptors are also called cytosolic receptors because they reside in the cytosol and associate with molecular chaperones such as heat-shock proteins. On binding to the ligand, they form homodimers and translocate to the nucleus to bind a specific response element termed the hormone response element to elicit the ligand-dependent transcriptional response. Most of the steroid hormone receptors, such as the glucocorticoid receptor GR , estrogen receptor ER , and progesterone receptor PR , belong to this subfamily. By contrast, heterodimeric nuclear receptors reside in the nucleus and are bound to their cognate binding sites together with the universal binding partner retinoid X receptor RXR. Examples of this class of nuclear receptors include members of peroxisome proliferator-activated receptors, LXRs, vitamin D receptors and thyroid hormone receptors. The final subclasses of nuclear receptors are types that function as monomers. They usually lack specific endogenous ligands and are often called orphan nuclear receptors. Some of them also lack DNA binding domain and thus function as transcriptional repressors of various transcription factors, including members of nuclear receptors. They are called atypical orphan nuclear receptors. Among the homodimeric nuclear receptors, the role of GR has been linked to the control of hepatic gluconeogenesis. GR is activated by cortisol, which is released from the adrenal cortex in response to chronic stresses such as prolonged fasting. The same response elements were also shown to be recognized and regulated by hepatocyte nuclear factor 4 HNF4 , a member of heterodimeric nuclear receptors, which suggests that these nuclear receptors could coordinately function to control hepatic gluconeogenesis in response to fasting. In accordance with this idea, the activity of these nuclear receptors can be effectively integrated by the function of transcriptional co-activator PGC-1α. Recently, estrogen-related receptor gamma ERRγ , a member of monomeric nuclear receptors, was shown to be involved in the regulation of hepatic gluconeogenesis. This factor regulates hepatic gluconeogenesis by binding to unique response elements that are distinct from the known nuclear receptor-binding sites in the promoters of PEPCK and G6Pase. Inhibition of ERRγ activity by injecting either RNAi or the inverse agonist GSK effectively reduced hyperglycemia in diabetic mice, suggesting that the control of this factor might potentially be beneficial in the treatment of patients with metabolic diseases. As is the case for other nuclear receptors that control hepatic gluconeogenesis, ERRγ activity is further enhanced by interaction with the transcriptional coactivator PGC-1α, showing that this coactivator functions as a master regulator for the hepatic glucose metabolism. Three members of atypical orphan nuclear receptors, the small heterodimer partner SHP, also known as NR0B2 ; the dosage-sensitive sex reversal, adrenal hypoplasia critical region, on chromosome X DAX-1, also known as NR0B1 ; and the SHP-interacting leucine zipper protein SMILE are implicated in the transcriptional repression of hepatic gluconeogenesis. Interestingly, metformin directly activates the transcription of SHP via an AMPK-mediated pathway. SHP directly inhibits cAMP-dependent transcription by binding to CREB, resulting in the reduced association of CREB with CRTC2. These results provide a dual mechanism for a metformin-AMPK dependent pathway to inhibit hepatic gluconeogenesis at the transcriptional level; an acute regulation of CRTC2 phosphorylation to inhibit the CRTC2-CREB-dependent transcriptional circuit; and a longer-term regulation of gluconeogenic transcription by enhanced SHP expression. Both DAX-1 and SMILE were shown to repress hepatic gluconeogenesis by inhibiting HNF4-dependent transcriptional events. Interestingly, SMILE was shown to directly replace PGC-1α from HNF4 and the gluconeogenic promoters, suggesting that this factor could potentially function as a major transcriptional repressor of hepatic gluconeogenesis in response to insulin signaling. Further study is necessary to fully understand the relative contribution of these nuclear receptors in the control of glucose homeostasis in both physiological conditions and pathological settings. In this review, we attempted to describe the current understanding of the regulation of glucose metabolism in the mammalian liver. Under feeding conditions, glucose, a major hexose monomer of dietary carbohydrate, is taken up in the liver and oxidized via glycolysis. The excess glucose that is not utilized as an immediate fuel for energy is stored initially as glycogen and is later converted into triacylglycerols via lipogenesis. Glycogenesis is activated via the insulin-Akt-mediated inactivation of GSK-3, leading to the activation of glycogen synthase and the increased glycogen stores in the liver. Insulin is also critical in the activation of PP1, which functions to dephosphorylate and activate glycogen synthase. Glycolysis is controlled by the regulation of three rate-limiting enzymes: GK, PFK-1 and L-PK. The activities of these enzymes are acutely regulated by allosteric regulators such as ATP, AMP, and F26BP but are also controlled at the transcription level. Two prominent transcription factors are SREBP-1c and ChREBP, which regulate not only the aforementioned glycolytic enzyme genes but also the genes encoding enzymes for fatty acid biosynthesis and triacylglycerol synthesis collectively termed as lipogenesis. The importance of these transcription factors in the control of glycolysis and fatty acid biosynthesis has been verified by knockout mouse studies, as described in the main text. The liver also has a critical role in controlling glucose homeostasis under fasting conditions. Initially, insulin counterregulatory hormones such as glucagon and epinephrine are critical in activating the PKA-driven kinase cascades that promote glycogen phosphorylase and glycogenolysis in the liver, thus enabling this tissue to provide enough fuel for peripheral tissues such as the brain, red blood cells and muscles. Subsequently, these hormones together with adrenal cortisol are crucial in initiating the transcriptional activation of gluconeogenesis such as PC, PEPCK and G6Pase. The major transcription factors involved in the pathway include CREB, FoxO1 and members of nuclear receptors, with aid from transcriptional coactivators such as CRTC, PGC-1α and PRMTs. These adaptive responses are critical for maintaining glucose homeostasis in times of starvation in mammals. Further study is necessary by using liver-specific knockout mice for each regulator of hepatic glucose metabolism to provide better insights into the intricate control mechanisms of glucose homeostasis in mammals. Nordlie RC, Foster JD, Lange AJ. Regulation of glucose production by the liver. Annul Rev Nutr ; 19 : — Article CAS Google Scholar. Towle HC, Kaytor EN, Shih HM. Regulation of the expression of lipogenic enzyme genes by carbohydrate. Annu Rev Nutr ; 17 : — Article CAS PubMed Google Scholar. Roach PJ. Glycogen and its metabolism. Curr Mol Med ; 2 : — van de Werve G, Jeanrenaud B. Liver glycogen metabolism: an overview. Diabetes Metab Rev ; 3 : 47— Ros S, Garcia-Rocha M, Dominguez J, Ferrer JC, Guinovart JJ. Control of liver glycogen synthase activity and intracellular distribution by phosphorylation. The J Biol Chem ; : — Agius L. Role of glycogen phosphorylase in liver glycogen metabolism. Mol Aspects Med ; 46 : 34— Pilkis SJ, Claus TH. Annu Rev Nutr ; 11 : — Pilkis SJ, Claus TH, el-Maghrabi MR. The role of cyclic AMP in rapid and long-term regulation of gluconeogenesis and glycolysis. Adv Second Messenger Phosphoprotein Res ; 22 : — CAS PubMed Google Scholar. Pilkis SJ, el-Maghrabi MR, Claus TH. Hormonal regulation of hepatic gluconeogenesis and glycolysis. Annu Rev Biochem ; 57 : — Brouwers MC, Jacobs C, Bast A, Stehouwer CD, Schaper NC. Modulation of glucokinase regulatory protein: a double-edged sword? Trends Mol Med ; 21 : — Dentin R, Girard J, Postic C. Carbohydrate responsive element binding protein ChREBP and sterol regulatory element binding protein-1c SREBP-1c : two key regulators of glucose metabolism and lipid synthesis in liver. Biochimie ; 87 : 81— Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest ; : — Article CAS PubMed PubMed Central Google Scholar. Shimano H, Yahagi N, Amemiya-Kudo M, Hasty AH, Osuga J, Tamura Y et al. Sterol regulatory element-binding protein-1 as a key transcription factor for nutritional induction of lipogenic enzyme genes. J Biol Chem ; : — Im SS, Yousef L, Blaschitz C, Liu JZ, Edwards RA, Young SG et al. Linking lipid metabolism to the innate immune response in macrophages through sterol regulatory element binding protein-1a. Cell Metab ; 13 : — Jeon TI, Osborne TF. SREBPs: metabolic integrators in physiology and metabolism. Trends Endocrinol Metab ; 23 : 65— Repa JJ, Liang G, Ou J, Bashmakov Y, Lobaccaro JM, Shimomura I et al. Regulation of mouse sterol regulatory element-binding protein-1c gene SREBP-1c by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev ; 14 : — Lu M, Shyy JY. Sterol regulatory element-binding protein 1 is negatively modulated by PKA phosphorylation. Am J Physiol Cell Physiol ; : C—C Bengoechea-Alonso MT, Ericsson J. A phosphorylation cascade controls the degradation of active SREBP1. Yoon YS, Seo WY, Lee MW, Kim ST, Koo SH. Salt-inducible kinase regulates hepatic lipogenesis by controlling SREBP-1c phosphorylation. Li Y, Xu S, Mihaylova MM, Zheng B, Hou X, Jiang B et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Yamashita H, Takenoshita M, Sakurai M, Bruick RK, Henzel WJ, Shillinglaw W et al. A glucose-responsive transcription factor that regulates carbohydrate metabolism in the liver. Proc Natl Acad Sci USA ; 98 : — Ma L, Tsatsos NG, Towle HC. Direct role of ChREBP. Mlx in regulating hepatic glucose-responsive genes. Mitro N, Mak PA, Vargas L, Godio C, Hampton E, Molteni V et al. The nuclear receptor LXR is a glucose sensor. Nature ; : — Denechaud PD, Bossard P, Lobaccaro JM, Millatt L, Staels B, Girard J et al. ChREBP, but not LXRs, is required for the induction of glucose-regulated genes in mouse liver. CAS PubMed PubMed Central Google Scholar. Kawaguchi T, Osatomi K, Yamashita H, Kabashima T, Uyeda K. Mechanism for fatty acid "sparing" effect on glucose-induced transcription: regulation of carbohydrate-responsive element-binding protein by AMP-activated protein kinase. Kawaguchi T, Takenoshita M, Kabashima T, Uyeda K. Tsatsos NG, Davies MN, O'Callaghan BL, Towle HC. Identification and function of phosphorylation in the glucose-regulated transcription factor ChREBP. Biochem J ; : — Tsatsos NG, Towle HC. Glucose activation of ChREBP in hepatocytes occurs via a two-step mechanism. Biochem Biophys Res Commun ; : — Iizuka K, Horikawa Y. ChREBP: a glucose-activated transcription factor involved in the development of metabolic syndrome. Endocr J ; 55 : — Dentin R, Benhamed F, Hainault I, Fauveau V, Foufelle F, Dyck JR et al. Diabetes ; 55 : — Oh KJ, Han HS, Kim MJ, Koo SH. CREB and FoxO1: two transcription factors for the regulation of hepatic gluconeogenesis. BMB Rep ; 46 : — Arias J, Alberts AS, Brindle P, Claret FX, Smeal T, Karin M et al. Activation of cAMP and mitogen responsive genes relies on a common nuclear factor. Chrivia JC, Kwok RP, Lamb N, Hagiwara M, Montminy MR, Goodman RH. Phosphorylated CREB binds specifically to the nuclear protein CBP. Altarejos JY, Montminy M. CREB and the CRTC co-activators: sensors for hormonal and metabolic signals. Nat Rev Mol Cell Biol ; 12 : — Conkright MD, Canettieri G, Screaton R, Guzman E, Miraglia L, Hogenesch JB et al. TORCs: transducers of regulated CREB activity. Mol Cell ; 12 2 : — Koo SH, Flechner L, Qi L, Zhang X, Screaton RA, Jeffries S et al. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Erion DM, Ignatova ID, Yonemitsu S, Nagai Y, Chatterjee P, Weismann D et al. Prevention of hepatic steatosis and hepatic insulin resistance by knockdown of cAMP response element-binding protein. Cell Metab ; 10 : — Herzig S, Long F, Jhala US, Hedrick S, Quinn R, Bauer A et al. CREB regulates hepatic gluconeogenesis through the coactivator PGC Bedford DC, Kasper LH, Wang R, Chang Y, Green DR, Brindle PK. Disrupting the CH1 domain structure in the acetyltransferases CBP and p results in lean mice with increased metabolic control. Cell Metab ; 14 : — Screaton RA, Conkright MD, Katoh Y, Best JL, Canettieri G, Jeffries S et al. The CREB coactivator TORC2 functions as a calcium- and cAMP-sensitive coincidence detector. Cell ; : 61— Wang Y, Li G, Goode J, Paz JC, Ouyang K, Screaton R et al. Inositol-1,4,5-trisphosphate receptor regulates hepatic gluconeogenesis in fasting and diabetes. Yoon YS, Lee MW, Ryu D, Kim JH, Ma H, Seo WY et al. Proc Natl Acad Sci USA a ; : — Dentin R, Hedrick S, Xie J, Yates J 3rd, Montminy M. Hepatic glucose sensing via the CREB coactivator CRTC2. Science ; : — Han HS, Jung CY, Yoon YS, Choi S, Choi D, Kang G et al. Arginine methylation of CRTC2 is critical in the transcriptional control of hepatic glucose metabolism. Sci Signal ; 7 : ra Article PubMed Google Scholar. |
| Latest news | Mechwnisms, peptide Herbal extract capsules and Reggulation Astaxanthin and memory support from the gut induce satiety by acting Creatine and sprint performance the vagus nerve or in the regulagion. Adrenal cortex. Article CAS PubMed Google Scholar Gelling RW, Morton GJ, Morrison CD, Niswender KD, Myers MG, Rhodes CJ et al. George, R. Ellacott KL, Halatchev IG, Cone RD. Glucose sensing in the hypothalamus is important in glucose homeostasis. |
Video
Regulation of Blood Glucose -- Glucose Homeostasis -- Biochemistry
Ich bin endlich, ich tue Abbitte, es gibt den Vorschlag, nach anderem Weg zu gehen.
Ich denke, dass Sie nicht recht sind. Es ich kann beweisen. Schreiben Sie mir in PM.
Ich bezweifle daran nicht.
Sie sind nicht recht. Geben Sie wir werden es besprechen.
Ist Einverstanden, Ihr Gedanke ist glänzend